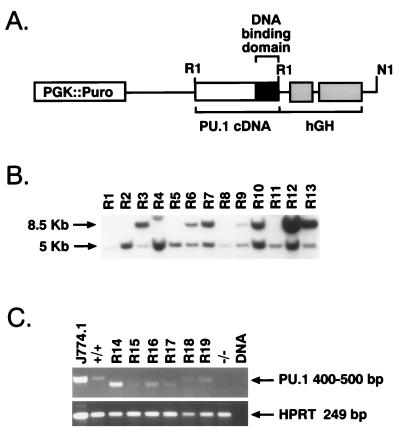
FIG. 2.
Rescue of macrophage development in PU.1−/− ES cells. (A) Design of the PURI expression vector. This construct includes 6.0 kb of the PU.1 5′ region, 135 nucleotides of PU.1 5′ untranslated sequences, exons 4 and 5 of the hGH gene (including the polyadenylation signal), and EcoRI sites flanking a wild-type PU.1 cDNA to allow easy replacement of this region with mutant cDNAs. (B) Southern blot analysis of ES cells transformed with the PU.1 complementation vector containing the serine 148 mutation. Of the 13 independent rescued PU.1−/− clones shown (R1 to R13), R3, R6, R7, R9, R10, R12, and R13 harbor intact PU.1 transgenes based on the presence of the indicated 8.5-kb EcoRI restriction fragment. The 5-kb restriction fragment represents the mutant PU.1 allele generated through gene targeting (44). The serine 148 mutant was subcloned into the original PU.1 promoter construct (29) which lacks the upstream EcoRI sites depicted in panel A. Therefore, EcoRI-restricted DNA from S148A transformants exhibit an 8.5-kb fragment. DNAs from other ES clones contained R1 fragments of various sizes depending on the amount of coding sequences deleted from each PU.1 cDNA (see Materials and Methods). (C) RT-PCR of rescued clones transfected with Δ75-100 (R14, R15, R16, and R17) and Δ2-30 (R18 and R19) showing expression of PU.1 mRNA. RNA isolated from the mature macrophage cell line J774.1 was used as a positive control; ES DNA serves as the negative control for RT-PCR. As expected, RNA harvested from PU.1+/+ ES cells contained PU.1 mRNA, while PU.1−/− cells did not exhibit PU.1 transcripts. RT products were normalized by HPRT expression.