Abstract
Current differentiation protocols for human induced pluripotent stem cells (hiPSCs) produce heterogeneous cardiomyocytes (CMs). Although chamber-specific CM selection using cell surface antigens enhances biomedical applications, a cell surface marker that accurately distinguishes between hiPSC-derived atrial CMs (ACMs) and ventricular CMs (VCMs) has not yet been identified. We have developed an approach for obtaining functional hiPSC-ACMs and -VCMs based on CD151 expression. For ACM differentiation, we found that ACMs are enriched in the CD151low population and that CD151 expression is correlated with the expression of Notch4 and its ligands. Furthermore, Notch signaling inhibition followed by selecting the CD151low population during atrial differentiation leads to the highly efficient generation of ACMs as evidenced by gene expression and electrophysiology. In contrast, for VCM differentiation, VCMs exhibiting a ventricular-related gene signature and uniform action potentials are enriched in the CD151high population. Our findings enable the production of high-quality ACMs and VCMs appropriate for hiPSC-derived chamber-specific disease models and other applications.
Subject terms: Induced pluripotent stem cells, Stem-cell differentiation
The authors demonstrate that CD151 expression distinguishes atrial from ventricular cardiomyocytes derived from induced pluripotent stem cells, while further showing the role of Notch signaling in mediating the process.
Introduction
Conventional methods used to differentiate human pluripotent stem cell-derived cardiomyocytes (hPSC-CMs) generate a mixture of different CM subtypes, including ventricular CMs (VCMs), atrial CMs (ACMs), and nodal-like cells1–3. Although such heterogeneity indicates the potential for producing an unlimited number of each subtype in vitro, it creates challenges for purifying chamber-specific cells for various applications such as disease modeling and drug testing.
Several groups have reported effective methods for differentiating hPSCs into each subtype. These methods mimic in vivo cardiac development by regulating several signaling pathways, such as WNT, BMP, Activin/Nodal, and retinoic acid (RA) signaling4–6. WNT signaling exerts a biphasic effect, promoting early cardiac development and inhibiting it later. Accordingly, appropriate inhibition of WNT signaling at the mesoderm stage leads to highly efficient differentiation of hPSC-CMs7–10. BMP signaling contributes to cardiac mesoderm differentiation in zebrafish, while Nodal signaling further promotes ventricular specification11–13. Embryos deficient in retinaldehyde dehydrogenase 2 (RALDH2), an enzyme involved in RA synthesis, show failed cardiac looping and defective atrial and sinus venosus development, thus indicating the importance of RA signaling for cardiovascular progenitor cell development14–16. RA signaling induces NR2F2 (COUP-TFII), a key transcriptional regulator, to direct the cell fate toward ACMs and directly regulates atrial- and ventricular-related genes involved in mouse cardiac development17 and the differentiation of human embryonic stem cells into ACMs5. These findings are applicable to hPSC-CMs, where reduced BMP, Activin/Nodal, and RA signaling promote the generation of hPSC-ACMs (atrial-biased differentiation), while higher BMP and Activin/Nodal signaling favor the generation of hPSC-VCMs18 (ventricular-biased differentiation). However, we noted the presence of undesired subtypes in each differentiation method (i.e., ventricular-like CMs were generated in atrial-biased differentiation and vice versa), implying that signal manipulation was insufficient for generating completely homogeneous subtype populations. In addition, studies have demonstrated differentiation methods using hPSCs with chamber-specific reporters19–21 or surface proteins to enrich each subtype6,22. Although assays using surface markers help to select CM subtypes, we still lack the markers to effectively purify functionally homogeneous chamber-specific hPSC-CMs applicable to multiple cell lines.
In the present study, we identified CD151, a member of the tetraspanin family, as a potential marker of chamber-specific CMs derived from human induced pluripotent stem cells (hiPSCs). Using an atrial differentiation protocol, we showed that the expression of atrial genes was higher in CD151low ACMs than in CD151high ACMs. Moreover, whereas CD151high ACMs contained CMs with immature atrial action potentials (APs) and upregulated NOTCH-related genes, only CD151low ACMs showed atrial APs. We also revealed that Notch signaling inhibition enhanced atrial differentiation via HEY2 suppression and that these ACMs exhibited significantly more functional properties than hiPSC-ACMs generated by the conventional protocol. Using a ventricular differentiation protocol, we demonstrated that the CD151high selection enriched for VCMs with high ventricular-related gene expression and activated mitosis to result in binucleation. These findings altogether indicate that CD151 can distinguish ACMs and VCMs derived from hiPSCs in a bivalent manner. Furthermore, we revealed a pivotal role of differential Notch signaling activities in CD151low and CD151high ACMs for atrial differentiation. Our study demonstrates that cardiac subtypes with molecular and functional properties of ACMs or VCMs can be efficiently obtained from hiPSCs-CMs.
Results
CD151 shows different expression patterns during hiPSC-ACMs and -VCMs differentiation
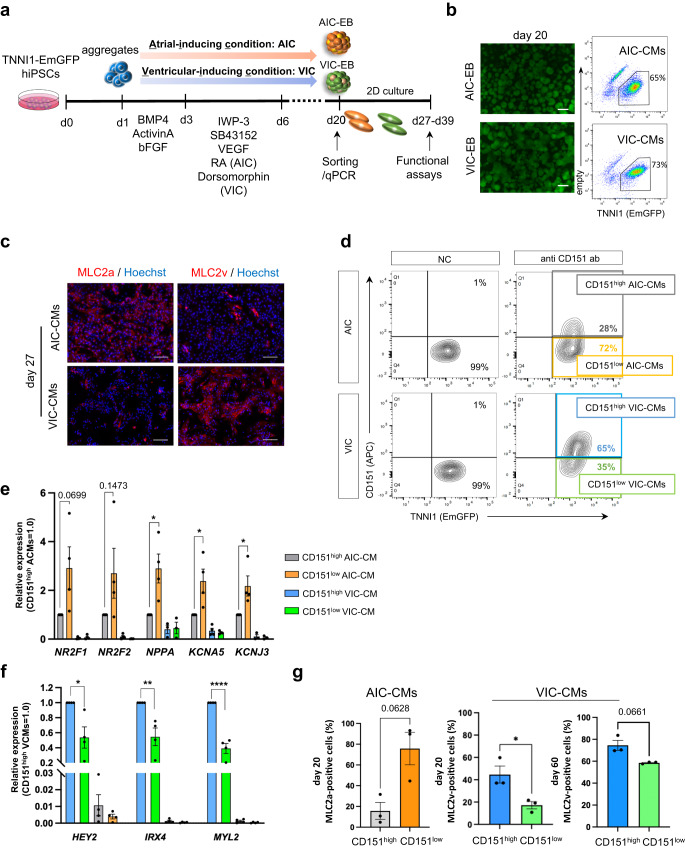
We differentiated ACMs and VCMs from hiPSCs based on a previously reported method6. ACMs were generated with a low concentration of agonists for BMP4 (3 ng/mL) and Activin A (4 ng/mL) in the early stage of embryoid body (EB) formation (days 1–3), followed by treatment with a combination of WNT inhibitor (IWP-3), Nodal inhibitor (SB43152), VEGF, and 0.5–1 μM RA during cardiac mesoderm induction (days 3–6). We refer to this differentiation condition as atrial-inducing condition (AIC; Fig. 1a). For differentiation into VCMs, hiPSCs were treated with a high concentration of BMP4 (10 ng/mL) and Activin A (6 ng/mL) on days 1–3, followed by IWP-3, SB43152, VEGF, and BMP4 inhibitor (Dorsomorphin) on days 3–6, which we refer to as ventricular-inducing condition (VIC; Fig. 1a).
Fig. 1. Cell surface marker screening for hiPSC-ACMs and -VCMs.
a Schematic diagram and experimental schedule of cardiac differentiation protocol for AIC and VIC. b Representative fluorescence and flow cytometry images of day 20 AIC- and VIC-CMs. Scale bars = 100 μm. (See also Fig. S1a). c Representative immunofluorescence images showing MLC2a, MLC2v, and Hoechst staining on day-27 AIC- and VIC-CMs. Scale bars = 100 μm. (See also Fig. S1b). d Representative flow cytometry images of day 20 AIC- and VIC-CMs stained with CD151 antibody. The cut-off between CD151high and CD151low cells was determined using unstained CMs as a negative control. The gate containing most negative control CMs (>99%) was set as the CD151low gate (See also Figs. S2a and S2d). e Relative expression of atrial-related genes in CD151high/low AIC- and VIC-CMs, compared to CD151high AIC-CMs. n = 4 independent differentiation experiments per group. Data are expressed as mean ± SEM. Statistical analysis was conducted between CD151high AIC-CM and CD151low AIC-CM using an unpaired two-tailed t test. *p < 0.05. (See also Figs. S2b and S2e). f Relative expression of ventricular-related genes in CD151high/low VIC- and AIC-CMs compared to that of CD151high VCMs. n = 4 independent differentiation experiments per group. Data are expressed as mean ± SEM. Statistical analysis was conducted between CD151high VIC-CM and CD151low VIC-CM using an unpaired two-tailed t test. *p < 0.05, **p < 0.01, and ****p < 0.0001 (See also Figs.S2c and S2f). g MLC2a-positive cells fraction (%) in CD151high/low AIC-CMs on day 20 (left). MLC2v-positive cells fraction (%) in CD151high/low VIC-CMs on day 20 and day 60 (right). n = 3 independent differentiation experiments per group. Data are expressed as mean ± SEM. Statistical analysis was conducted using an unpaired two-tailed t test. *p < 0.05.
First, we utilized the TNNI1-EmGFP/TNNI3-mCherry double reporter hiPSC line generated in our previous study23. This line enables the visualization of CMs exhibiting green fluorescence due to the placement of EmGFP downstream of TNNI1 (Fig. 1b). In this study, we concentrated on the TNNI1-EmGFP marker (referred to as “TNNI-EmGFP” henceforth). On day 27, TNNI1+ cells in AIC- and VIC-EBs predominantly expressed MLC2a and MLC2v, respectively (Fig. 1c). We confirmed that AIC- and VIC-EBs also contained MLC2v- and MLC2a-positive CMs, respectively, suggesting that AIC and VIC resulted in a mixture of VCM-like and ACM-like cells. To determine whether the heterogeneity resulting from these differentiation protocols is reproducible, we differentiated and purified hiPSC-CMs from 1390C1, an independent hiPSC line, using the pan-CM marker SIRPA and other lineage markers (CD31, CD49a, CD90, and CD140b)24 (Fig. S1a). Immunofluorescence analysis indicated that most AIC-CMs were positive for MLC2a but contained some MLC2v+ CMs, whereas VIC-CMs were predominantly positive for MLC2v but also contained MLC2a+ CMs (Fig. S1b). Despite long-term culture, MLC2v+ and MLC2a+ CMs were still present in day 60 AIC and VIC cultures, respectively (Fig. S1c). These observations indicated that even protocols with optimized concentrations of BMP4, Activin A, and RA for chamber-specific CM generation showed a mix of CM subtypes.
To enhance CM subtype specificity, we sought to identify differentially expressed markers present in AIC- and VIC-CMs. We performed screening of 212 cell surface markers with the panel of antibodies by comparing the expression patterns of CMs induced by AIC and VIC (Fig. S1d). We eliminated cell surface antigens whose expressions were affected only by BMP (3 ng/mL) and Activin A (4 ng/mL) without RA since RA signaling is critical for differentiation into ACMs. We found five cell surface antigens with different expression patterns between AIC- and VIC-CMs (Fig. S1e). Flow cytometry analysis showed that CD57, CD71, and CD98 were expressed in AIC and AIC without RA, thus disqualifying them as subtype-specific markers (Fig. S1e (i–iii)). Although the expression pattern of SSEA-4 in AIC was distinct from the other conditions (Fig. S1e iv), most AIC-CMs were SSEA-4 negative (Fig. S1f). Finally, CD151 was highly expressed in VIC- and AIC without RA-CMs but not in AIC-CMs (Fig. S1ev), and thus we focused on CD151 as a candidate marker to distinguish AIC-CMs from VIC-CMs.
We analyzed the expression levels of subtype-related genes to determine whether CD151 can separate ACMs from VCMs. We sorted CD151high/low CMs from AIC- and VIC-EBs (Fig. 1d) to analyze the expressions of atrial- and ventricular-related genes via qPCR. AIC-CD151low CMs (CD151low ACMs) highly expressed atrial-related genes, such as NPPA, KCNA5, and KCNJ3, compared to AIC-CD151high CMs (CD151high ACMs). In contrast, the expression levels of those genes in VIC-CMs were low regardless of CD151 selection (Fig. 1e). Furthermore, the expression levels of ventricular-related genes (HEY2, IRX4, and MYL2) in VIC-CD151low CMs (CD151low VCMs) or CD151high/low ACMs were significantly lower than those in VIC-CD151high CMs (CD151high VCMs) (Fig. 1f). To validate these findings in other hiPSC lines, we sorted CD151high/low CMs from AIC- and VIC-EBs using SIRPA and other lineage markers (Figs. S2a and S2d) and confirmed that atrial- and ventricular-related gene expression patterns in observed CD151high/low ACMs and VCMs were similar to those derived from TNNI1-EmGFP reporter hiPSCs (Figs. S2b, S2c, S2e, and S2f). On day 27, MLC2a-positive CMs were clearly detected among CD151low ACMs. Whereas CD151low VCMs showed some MLC2a-positive cells, CD151high VCMs did not (Fig. S2g). Furthermore, we detected 93% and 36% of MLC2a-positive cells in CD151low - and CD151high AIC-CMs, respectively by flow cytometric analysis on day-20 TNNI1-EmGFP hiPSCs, and 45% and 17% of MLC2v-positive cells in CD151high- and CD151low VIC-CMs, respectively (Fig.1g). These findings suggest that CD151low AIC-CMs and CD151high VIC-CMs were enriched ACMs and VCMs, respectively.
CD151high/low CMs exhibited differential electrophysiological properties
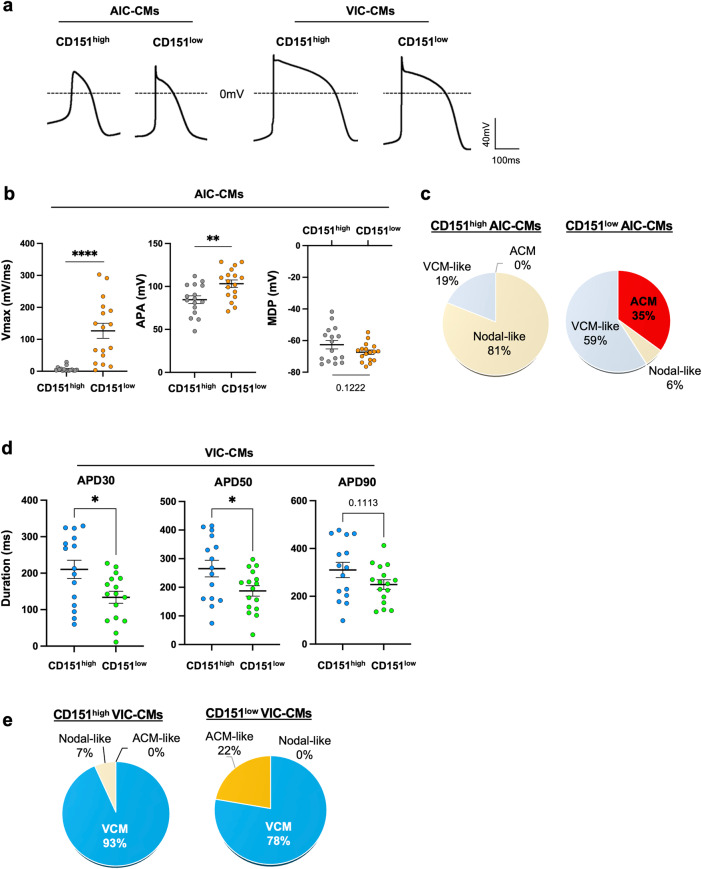
Next, we investigated the electrophysiological properties of CD151high/low ACMs and VCMs by measuring action potentials (APs) using a patch-clamp method (Fig. 2a). In AIC, CD151high ACMs showed a significantly slower maximum upstroke velocity (Vmax) than CD151low ACMs, which is a characteristic feature of nodal-like CMs (Fig. 2b). Furthermore, although there was no significant difference between the maximal depolarization potentials (MDPs) of CD151high ACMs and CD151low ACMs, the action potential amplitude (APA) of CD151low ACMs was increased compared to that of CD151high ACMs (Fig. 2b). To determine the fraction of cells showing atrial type APs (AP duration at 30% of repolarization (APD30) / AP duration at 90% of repolarization (APD90) < 0.3 and Vmax > 10), we classified CD151high/low cells into each CM subtype (ventricular-type - APD30/90 > 0.3 and Vmax > 10; nodal-type - Vmax ≤ 10). While no atrial type APs and 81% of nodal-like CMs were detected in CD151high ACMs, ACMs exhibiting atrial type APs were found only in CD151low population, and they exhibited significantly smaller APD30/90 than CD151high VCMs (Figs. 2c and S3b). Although the proportion of atrial-type APs was only 35% (Fig. 2c and Supplementary Table 1), these data show that ACMs were enriched in CD151low ACMs, consistent with the high expression of atrial-related genes in CD151low populations (Figs.1e, S2b, and S2e).
Fig. 2. Electrophysiological properties of CD151high/low ACMs and VCMs.
a Representative APs recorded in CD151high/low ACMs from AIC-CMs and CD151high/low VCMs from VIC-CMs. b Electrophysiology measurements of Vmax, APA, and MDP in CD151high ACMs (n = 16) and CD151low ACMs (n = 17) from three independent differentiation experiments. Data are presented as mean ± SEM. Statistical analysis was conducted using an unpaired two-tailed t test. **p < 0.01 and ****p < 0.0001. c The proportions of AIC-CMs with atrial (ACM; APD30/90 < 0.3, Vmax > 10), ventricular-like (VCM-like; APD30/90 ≥ 0.3, Vmax > 10), and nodal-like APs (Vmax ≤ 10) in CD151high ACMs (n = 16) and CD151low ACMs (n = 17). d Electrophysiology measurements of APD30, APD50, and APD90 in CD151high VCMs (n = 15) and CD151low VCMs (n = 16) from three independent differentiation experiments. Data are presented as mean ± SEM. Statistical analysis was conducted using an unpaired two-tailed t test. *p < 0.05. (See also Fig. S3b). e The proportions of VIC-CMs with ventricular (VCM), atrial-like (ACM-like), and nodal-like APs in CD151high (n = 15) and CD151low VCMs (n = 18). The criteria are described in (c).
Although the levels of Vmax, APA, and MDP were similar in both CD151high and CD151low VCMs under VIC (Fig. S3a), APD30 and APD50 were significantly shorter in CD151low VCMs than CD151high VCMs (Fig. 2d). Additionally, we found that while CD151low VCMs contained cells showing atrial-type APs (22%), CD151high VCMs only displayed ventricular-type APs (93%) (Fig. 2e; Supplementary Table 1), consistent with the shorter APDs seen in CD151low VCMs (Fig. 2d). These data demonstrate that hiPSC-VCMs were purified by CD151 expression.
CD151 does not directly regulate subtype differentiation
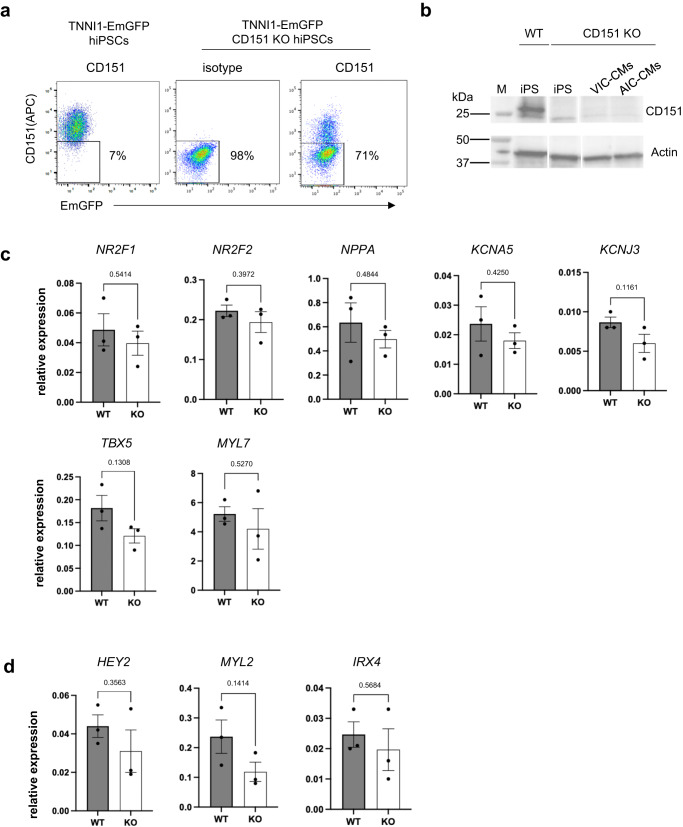
To determine whether CD151 directly regulates atrial and ventricular differentiation, we deleted CD151 from the TNNI1-EGFP hiPSC line using CRISPR-Cas9. We sorted CD151 negative cells from gRNA-transfected cells (Fig. 3a) and confirmed that CD151 was not expressed in generated knockout (KO) hiPSC line (CD151 KO hiPSCs) at the protein level (Figs. 3b and S4a). Genomic validation revealed that a frameshift was induced with 100% efficiency in CD151 KO iPSCs (Fig. S4b). Furthermore, there were no mutations at the predicted off-target sites in exons (Fig.S4c). Subsequently, we cultured CD151 KO hiPSCs and differentiated them into the AIC- and VIC-CMs. Both CMs did not express CD151 at the protein level (Figs. 3b and S4d). Using this CD151 KO line, we analyzed the expression of atrial and ventricular marker genes in AIC- and VIC-CMs on day 20, respectively, and compared the expression levels with those in WT CMs. No significant changes in atrial (Fig. 3c) or ventricular (Fig. 3d) marker gene expression were observed in CD151 KO cells, indicating that CD151 does not regulate the atrial and ventricular differentiation.
Fig. 3. CD151 KO hiPSC-derived AIC-CMs and VIC-CMs.
a Generation of the CD151 KO hiPSC line. Representative FACS plot of Cas9 alone-transfected hiPSCs (left), CD151 KO gRNA-transfected hiPSCs with the isotype control (middle) and CD151 stained sample (right). About 71% of the transfected cells were overlapped with the isotype control. This population was sorted and harvested. b Western blots analysis of CD151 expression. The samples were WT-hiPSCs, CD151 KO gRNA transfected-hiPSCs, VIC-CMs, and AIC-CMs. M shows the molecular weight marker. Top panel: anti-CD151 antibody; bottom panel: anti-Actin as loading control. The sample lanes were re-arranged of non-adjacent lanes in the original blot images (See also Fig. S4a). Atrial marker expressions in WT and CD151 KO hiPSC-derived AIC-CMs (c) and ventricular marker expressions (d) in WT and CD151 KO hiPSC-derived VIC-CMs. n = 3 independent differentiation experiments per group. Data are expressed as mean ± SEM. Statistical analysis was conducted using an unpaired two-tailed t test.
Notch signaling inhibition promotes atrial differentiation from hiPSCs
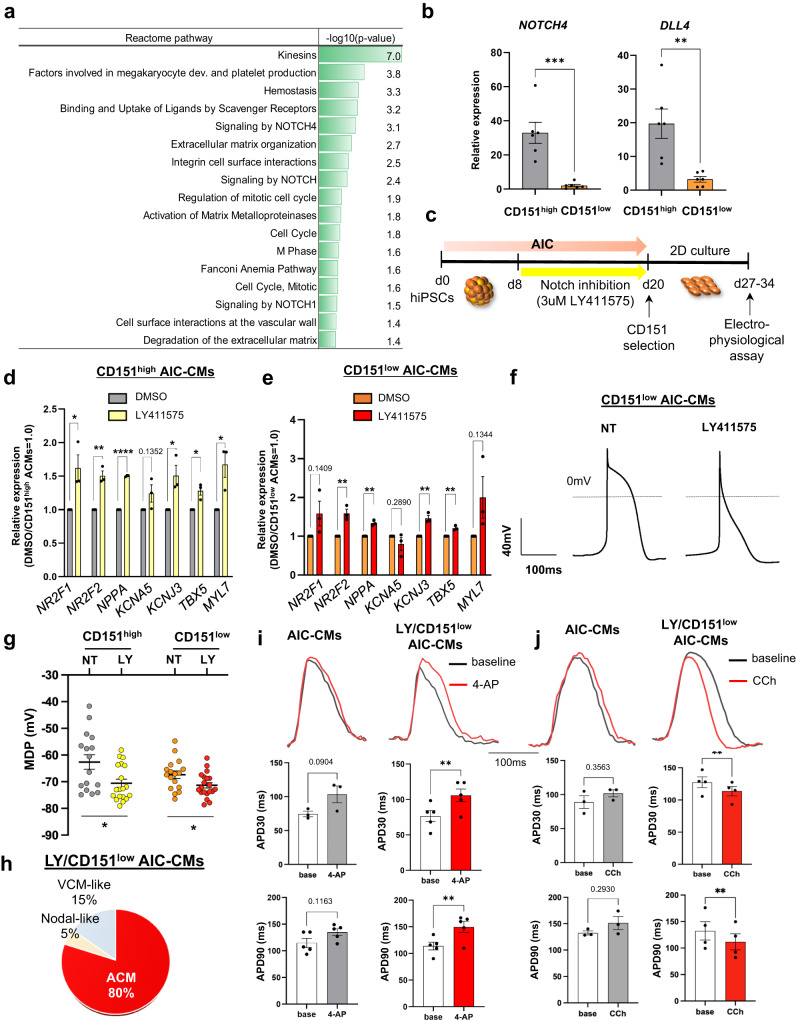
Next, we investigate whether CD151 expression is reflected by difference in signaling molecules and pathways regulating subtype. To examine this possibility in detail, we performed RNA sequencing (RNA-seq) of CD151high/low ACMs and VCMs. In principal component analysis (PCA), the first component (PC1), which accounted for 55.99% of the variance in gene expression, separated AIC- and VIC-CMs, and the second component (PC2, 13.41% of the variance) separated the expression levels of CD151 in AIC- and VIC-CMs (Fig. S5a). We performed GO and Reactome pathway analyses with highly correlated PC2 ( | factor loadings of PC2 | > 0.8) in differentially expressed genes (DEGs) between CD151high ACMs and CD151low ACMs (Supplementary Data 1). GO enrichment analysis and hierarchical clustering revealed an enrichment of cell adhesion-related genes (Figs. S5b and S5c). Additionally, Reactome pathway analysis revealed a prominent molecular signature by the Notch signaling pathway (Fig. 4a). The expression of NOTCH4 and its ligands (DLL4 and DLK1) made a high contribution to PC2 (loadings were 0.84, 0.90, and 0.90, respectively) (Fig. S5d), suggesting that the expression of NOTCH4, DLL4, and DLK1 was highly correlated with CD151 expression. To validate our findings, we examined the expression levels of NOTCH4 and DLL4 in CD151high/low ACMs via qPCR and found that their expression levels in CD151high ACMs were significantly higher than those in CD151low ACMs (Fig. 4b).
Fig. 4. Notch signaling inhibition promotes atrial differentiation from hiPSCs.
a Reactome pathway analysis in AIC-CMs. Three Notch signaling pathways were included in the 17 pathways with a significance level of p < 0.05. b Relative expression of NOTCH4 and DLL4 genes in CD151high ACMs compared to CD151low ACMs. n = 6 independent differentiation experiments per group. Data are presented as mean ± SEM. Statistical analysis was conducted using an unpaired two-tailed t test (See also Fig. S5d). **p < 0.01 and ***p < 0.001. c Schematic diagram of the assay schedule for Notch inhibition treatment for AIC differentiation. d Relative expression of atrial-related genes in CD151high ACMs differentiated with LY411575 or DMSO as vehicle control. n = 3 independent differentiation experiments per group. Data are presented as mean ± SEM. Statistical analysis compared two treatments for each gene using an unpaired two-tailed t test. *p < 0.05, **p < 0.01, and ****p < 0.0001. e Relative expression of atrial-related genes in CD151low ACMs differentiated using LY411575 or DMSO as vehicle control. n = 3 independent differentiation experiments per group. Data are expressed as mean ± SEM. Statistical analysis compared two treatments for each gene using an unpaired two-tailed t test. **p < 0.01. f Representative AP waveforms of CD151low ACMs differentiated with vehicle (DMSO) or LY411575 treatment. g Electrophysiological measurements of MDP in CD151high/low ACMs differentiated with vehicle or LY411575 treatment. For CD151high ACMs, DMSO (n = 16) and LY411575 treatment (n = 19); for CD151low ACMs, DMSO (n = 17) and LY411575 treatment (n = 20). Data are presented as mean ± SEM. Statistical analysis was conducted using an unpaired two-tailed t test. *p < 0.05. h The proportion of atrial (ACM), VCM-like, and nodal-like APs in CD151low ACMs differentiated with LY411575. i Representative APs of AIC-CMs and LY/CD151low ACMs detected using FluoVolt dye before and after 4-AP treatment or (j) CCh treatment. APD30 and APD90 were analyzed using the averages of 10 APs at baseline and after drug treatment under 4 Hz pacing. n = 3–5 independent differentiation experiments. Data are expressed as mean ± SEM. Statistical analysis was conducted using a paired two-tailed t test. **p < 0.01.
Notch signaling is regulated via direct cell-cell interactions between cells expressing the ligand and cells expressing the Notch receptor. Therefore, based on our findings, we hypothesized that Notch signaling activated by cell-cell interactions in CD151high ACMs negatively regulated atrial differentiation in the AIC. To determine whether Notch signaling inhibits atrial differentiation under AIC, we treated AIC-EBs with a gamma-secretase inhibitor, LY411575, on days 8–20 of differentiation (Fig. 4c) because NOTCH4 is highly expressed in AIC-EBs after day 8 (Fig. S5e). On day 20, the ratio of CD151high ACMs to CD151low ACMs remained unchanged with or without LY411575 treatment (Fig. S5f), suggesting that CD151 expression is not regulated by Notch signaling. We sorted TNNI1+ CMs and then analyzed the expression of subtype marker genes. The qPCR results showed that LY411575 treatment increased the expression of atrial-related genes in both CD151high and CD151low ACMs (Figs. 4d and e), confirming that the inhibition of Notch signaling promotes atrial differentiation in AIC-EBs. Moreover, HEY2, a known target of the Notch signaling pathway and a suppressor of atrial-related gene expression25, was downregulated by LY411575 (Fig. S5g). Although the causal role of HEY2 in atrial differentiation requires further investigation, our data highlight the potential involvement of HEY2 in mediating the gene expression changes downstream of Notch signaling, the inhibition of which promotes atrial specification.
To clarify whether Notch inhibition contributes to atrial differentiation in CD151high/low ACMs, we analyzed APs using patch-clamp experiments. The waveforms of LY411575/CD151low ACMs showed no plateau phase (Fig. 4f), and MDPs were significantly more hyperpolarized than those of the untreated groups (Fig. 4g). APAs and Vmax of LY411575/CD151high ACMs were significantly increased (Figs. S5h and S5i). Furthermore, 80% of the LY411575/CD151low ACMs were found to exhibit atrial-type APs (Fig. 4h). These results demonstrated that ACMs were generated and enriched with high efficiency in the CD151low ACMs treated with LY41157. Finally, to verify the functional properties of LY411575/CD151low ACMs, the drug responsiveness of CM sheets was measured with the atrial-specific ultra-rapid outward current (IKur) blocker, 4-aminopyridine (4-AP), and acetylcholine-induced current (IAch) agonist, carbachol (CCh), using a membrane potential-sensitive fluorescent dye (FluoVolt). To reveal the practical advantage of combining CD151 selection following Notch inhibition, we compared the responsiveness of LY411575/CD151low ACMs and conventional AIC-CMs. LY411575/CD151low ACMs showed significantly prolonged and notably shortened APD following 4-AP (Fig. 4i) and CCh treatments (Fig. 4j), respectively. In contrast, AIC-generated cells did not respond to these reagents (Figs. 4i and j). These data indicated that LY411575/CD151low ACMs were more functional as ACMs compared to conventionally differentiated ACMs. Altogether, these findings suggest that Notch signaling contributes to atrial differentiation potentially by regulating HEY2 expression (Fig. S5j), and an approach combining Notch inhibition (which promotes the differentiation of ACMs) and CD151-based selection significantly improves the purification of functional ACMs.
CD151high VCM gene expression profile and binucleation are indicative of advanced differentiation
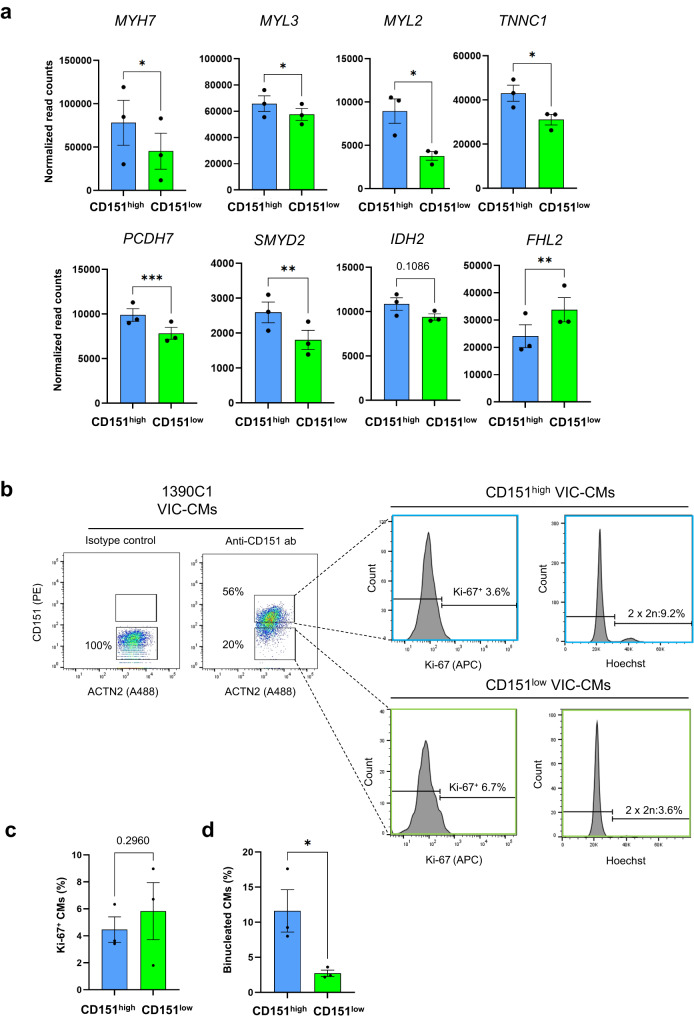
Under VIC, we demonstrated that CD151high VCMs are a purified population of VCMs without contamination by CMs exhibiting atrial-type APs. To identify the gene signatures of VCM differentiation dependent on CD151 expression, we analyzed RNA-seq data and identified MYL2 and HEY2 as DEGs in VIC-CMs (Supplementary Data 2). To determine whether CD151high VCMs express higher levels of other ventricular-related genes, we analyzed the expression of eight ventricular marker genes expressed in human ventricular tissues26. Six genes (MYH7, MYL3, MYL2, TNNC1, PCDH7, and SMYD2) were significantly upregulated in CD151high VCMs (Fig. 5a). Next, we performed GO and Reactome pathway analyses, which highlighted cell cycle and mitotic pathways (Figs. S6a and S6b). During human heart development, CMs stop proliferating after birth, and nuclear division occurs in postnatal CMs without cytokinesis, leading to the binucleation of CMs. To investigate whether mitotic progression in CD151high VCMs contributes to the proliferation or binucleation of CMs, we analyzed the expression of Ki-67, a proliferation marker. Flow cytometric analysis revealed that the percentages of Ki-67+ cells in CD151high VCMs and CD151low VCMs were 4.5% ± 0.9% and 5.8% ± 2.1%, respectively, indicating no significant difference between the two groups (Figs. 5b and c). Next, we investigated if CD151high VCMs contained more binuclear cells because binucleated CMs are found in adult mice and as human hearts maturate27,28. Flow cytometric analysis using Hoechst revealed that CD151high VCMs contained significantly more binucleated CMs (11.6% ± 3.0%) than CD151low VCMs (2.7% ± 0.4%) (Figs. 5b and 5d). Increasing binucleated CMs without cell proliferation was also observed using another hiPSC line (Figs. S6c and S6d). These results indicated that CD151high VCMs contain more binuclear CMs, accounting for the activation of cell cycle- and mitosis-related genes in CD151high VCMs. Considered together, CD151high VCMs are a population undergoing advanced VCM differentiation.
Fig. 5. CD151high VCMs expressed higher levels of ventricular genes and contained binuclear CMs.
a The expression levels of eight ventricular marker genes in CD151high/low VCMs. Data represent normalized read counts from RNA-seq data. n = 3 independent experiments per group. The data are expressed as mean ± SEM. Statistical analysis was conducted using an unpaired two-tailed t test. *p < 0.05, **p < 0.01, and ***p < 0.001. b Representative flow cytometry images of day-20 VIC-CMs stained with ACTN2, CD151, Ki-67, and Hoechst derived from the 1390C1 hiPSC line. c The percentage of Ki-67+ cells in CD151high VCMs and CD151low VCMs derived from the 1390C1 hiPSC line. n = 3 independent experiments per group. Data are expressed as mean ± SEM. Statistical analysis was conducted using an unpaired two-tailed t test. d Percentage of binuclear CMs in CD151high VCMs and CD151low VCMs derived from the 1390C1 hiPSC line. n = 3 independent differentiation experiments per group. Data are expressed as mean ± SEM. Statistical analysis was conducted using an unpaired two-tailed t test. *p < 0.05.
Discussion
In this study, we demonstrated hiPSC-ACMs to enrich in the CD151low population of AIC-CMs, and conversely, the CD151high VIC-CM population to contain enriched hiPSC-VCMs. Moreover, we identified CD151 as an indicator of Notch signaling and showed for the first time that its inhibition enhances atrial differentiation from hiPSCs. Under VIC, CD151high VCMs were purified binuclear VCMs with high ventricular genes expression, while CD151low VCMs exhibited low ventricular gene expression and contained some atrial-like CMs.
Chamber-specific differentiation protocols5,6, the identification of chamber-specific markers6,22, and the generation of chamber-specific reporter hPSCs19–21 have been used to acquire each CM subtype selectively. However, current differentiation protocols, including the conventional AIC and VIC we used here, produce heterogeneous populations of hiPSC-CMs with varying degrees of maturity and quality. Although purification methods that use chamber-specific reporter hPSCs are very efficient, they cannot be applied widely to non-reporter cell lines. Veevers et al. showed that although hESC-VCMs were enriched in CD77+/CD200- population, CD77 expression depended on the cell line used22. Indeed, CD77 did not react with hiPSC-CMs in our screening panel (Fig. S1d). Here, we found that CD151, in contrast, is a unique and robust marker of chamber specification in both ACMs and VCMs, and its differential expression can be applied to isolate functional CM subtypes derived from multiple hiPSC lines. We demonstrated that CD151 can also enrich the CD151low AIC-CM population that exhibits a high expression of atrial genes and the CD151high VIC-CMs, characterized by a high expression of ventricular genes, in monolayer differentiation (Figs. S7a and S7b). Our approach may help advance the biomedical applications of hiPSC-CMs.
CD151 belongs to the tetraspanin superfamily, which is involved in cellular processes, such as cell differentiation, proliferation, adhesion, migration, and intracellular signaling via various membrane proteins29. The known functions of CD151 have been studied primarily in oncology relating to invasion and metastasis. However, its role in cardiac development, differentiation, and chamber specification is completely unknown. We revealed a difference in CD151 expression between hiPSC-ACMs and -VCMs at 3–4 weeks post-differentiation. We also found that CD151 expression was upregulated in both AIC- and VIC-CMs as differentiation progressed (Fig. S7c). Consistent with this, the ratios of CD151high to CD151low population increased in a time-dependent manner in both AIC- and VIC-CMs (Fig. S7d). To investigate whether in vivo atrial and ventricular tissues show a similar difference in CD151 expression during development, we analyzed CD151 expression at the single-cell level in mice and human left atrium (LA) and ventricle (LV) using published data30,31. In line with our in vitro results, CD151 expressed higher in LV than in LA at mouse E10.5, human 7w, and 13w. However, such expression differences diminished during development (Fig. S7e). We also examined whether CD151low AIC-CMs and CD151high VIC-CMs could maintain their subtype phenotypes during extended culture. On day 60, no significant disparity in the expression of atrial marker genes was observed between high/low cells in AIC-CMs. Taken together, these findings indicate that CD151 is a transient marker of CM differentiation corresponding to embryonic heart organogenesis, and suggest that CD151 might serve as a marker of maturation level in AIC-CMs around day 20; CD151low AIC-CMs were more mature than CD151high AIC-CMs. The capacity to select more matured cells at an early stage will hold significant promise for future applications in disease models. In contrast, regarding VIC-CMs, while there was no difference in MYL2 expression between CD151 high/low cells on day 60, HEY2 and IRX4 expression levels were still reduced in CD151low VIC-CMs compared to CD151high VIC-CMs (Fig. S7f). Since MYL2 expression increased during maturation, this suggests that CD151 may mark maturation state, at the same time, CD151high and CD151low VIC-CMs may alternatively represent cell subpopulations with distinct molecular characteristics. Due to their high expression of ventricular marker genes, CD151high VIC-CMs are a more suitable choice for further investigation for disease modeling and drug testing.
Integrin is a family of proteins reported previously to interact with CD151. Transcriptome analysis showed that ITGA6 expression in AIC-CMs was higher than in VIC-CMs (Fig. S7g) and was inversely correlated with CD151 expression under AIC. These results substantiate a report that distinguished mouse ACMs by their ITGA6 expression32. However, flow cytometry using the CD49f (ITGA6) antibody indicated that CD49f did not help to differentiate between VIC- and AIC-CMs at the protein level because most VIC- and AIC-CMs were CD49f-positive (Fig. S7h). Our screening showed that two other tetraspanins, CD63 and CD81, were expressed in TNNI1-EmGFP reporter hiPSC-CMs, albeit at lower levels than that of CD151, but their levels were similar between AIC- and VIC-CMs (Fig. S1d). These results suggest CD151-specific signaling may be activated in AIC- and VIC-CM differentiation.
In this study, we found that NOTCH4 and its ligands, DLL4 and DLK1, were highly correlated with the expression of CD151 in AIC-CMs. Notch signaling is activated by cell-cell adhesion due to the binding of Notch receptors to their ligands. Hierarchical clustering analysis revealed that the GO term enriched by DEGs in CD151high ACMs was cell-cell adhesion (Fig. S5c). These findings suggest that CD151 expression is associated with Notch signaling activation induced by cell-cell adhesion. Although the Notch signaling pathway is highly conserved in multicellular organisms and regulates cell fate decisions in various differentiation processes, little is known about its role in atrial differentiation from hPSCs. In the present study, CD151high ACMs expressed higher levels of NOTCH4 and DLL4 than CD151low ACMs and showed nodal-like APs with slow Vmax in patch-clamp experiments. To determine if CD151high ACMs are similar to sinoatrial node (SAN) CMs, we examined the expression levels of SAN-specific genes and found no changes in CD151high ACMs (Fig. S7i), indicating CD151high ACMs were molecularly distinct from SAN CMs. Moreover, a previous study reported that the APs of immature hiPSC-derived CMs resemble those of SAN CMs based on the expression of the funny current (If) channel and low levels of the inward rectifier potassium current (Ik1) channel33. Thus, these results collectively suggested that CD151high ACMs were immature ACMs with high Notch signaling activity. A previous report showed that transient Notch activation in hPSC-derived cardiac mesoderm promotes immature cardiomyocytes with no change in their subtypes34. Though the time points of Notch activation or inhibition are different between that report and our study, Notch signaling may be critical for regulating the maturation of both VCMs and ACMs rather than cell fate.
Furthermore, Notch signaling inhibition with LY411575 increased the number of ACMs showing atrial-type AP waveforms and elevated expression of atrial-related genes accompanied by reduced HEY2 expression (Figs. 4d–f, and S5g). These results indicate the inhibition of Notch signaling has the potential to promote atrial differentiation by reducing the expression of HEY2, a downstream target of Notch signaling and a repressor of atrial-related genes, such as TBX5, NPPA, and MYL725 (Fig. S5j). In contrast, Notch activation after cell fate determination may not contribute to VCM differentiation efficiency since Notch signaling-related genes were expressed lowly in VIC-CMs (Fig. S5d).
Under VIC, we demonstrated that whereas VCMs express higher levels of ventricular marker genes and contain more binuclear cells when enriched in the CD151high VCM population, the CD151low VCM population had many atrial-like CMs (22%), potentially due to insufficient HEY2 expression to prevent ventricular differentiation in CD151low cells. Furthermore, multinucleated CMs constitute approximately 20–30% of CMs in the human heart28, and there is a substantial difference in binucleation levels exists between VCMs and ACMs, as shown by the relative proportion of binuclear CMs in the mouse ventricle and atria, at approximately 80% and 14%, respectively35. Altogether, although species differences must be taken into consideration, the binucleation of CMs during development may be a prominent feature of VCMs.
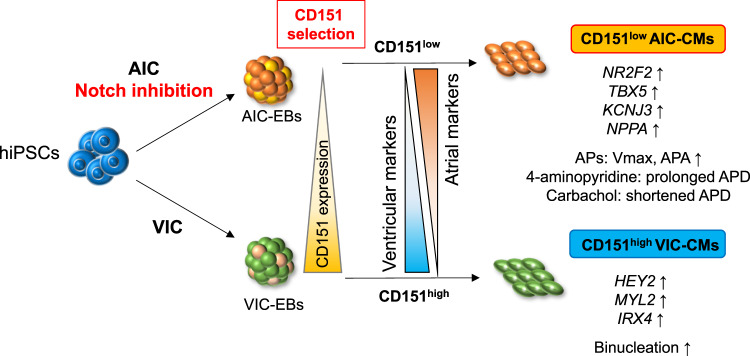
In conclusion, we demonstrated that CD151 expression is correlated with Notch signaling and mitotic activity, making it a helpful tool for selecting highly differentiated and functional hiPSC-ACMs and -VCMs (Fig. 6). Although this study did not elucidate the direct relationship between CD151 and the signaling pathways involved, we were able to obtain functional ACMs and VCMs from hiPSCs using a simple method, which represents a significant advance in the field. Furthermore, the mechanistic understanding of atrial differentiation from hiPSCs mediated by Notch signaling clarified here may help enhance the quality of ACMs during cardiac differentiation.
Fig. 6. CD151 expression-based hiPSC-ACM and VCM purification.
A schematic diagram depicting our work to distinguish AIC-CMs and VIC-CMs derived from hiPSCs at days 20–30 based on CD151 expression. Under AIC, Notch inhibition is critical for generating ACMs with high efficiency.
Methods
Human iPSC maintenance
The hiPSC lines (TNNI1-EmGFP reporter, 1390C1, and 409B2) were established in our institute23. The TNNI1-EmGFP reporter and 1390C1 hiPSC lines were maintained on an iMatrix-511 (Nippi)-coated dish in AK02N medium (Ajinomoto) as previously described23. The 409B2 hiPSC line was maintained on SL10 feeder cells (REPROCELL) in Repro Stem medium (REPROCELL) supplemented with 5 ng/mL bFGF (REPROCELL) and penicillin/streptomycin (Sigma), as previously described36. Human iPSC studies were approved by the relevant ethical committee.
Differentiation of hiPSCs into VCMs and ACMs
To initiate cardiac differentiation of the TNNI1-EmGFP reporter and 1390C1 hiPSC lines, we used previously described protocols with some modifications23,24. Briefly, hiPSCs were dissociated into single cells with Accumax (STEMCELL TECHNOLOGIES), and EBs were generated using a 6-well ultra-low attachment plate at 2 × 106 cells/well in 1.5 mL/well StemPro-34 medium (Thermo Fisher Scientific) containing 2 mM L-glutamine (Sigma), 50 μg/mL ascorbic acid (AA, sigma), 0.4 mM monothioglycerol (MTG, Sigma), 150 μg/mL transferrin (Wako), 0.5% Matrigel (Corning), 10 μM ROCK inhibitor Y-27632 (Wako), and 2 ng/mL BMP4 (R&D). On day 1 of ACM differentiation, 1.5 mL StemPro-34 medium with the above supplements (without Y-27632 or Matrigel), 10 ng/mL bFGF (R&D, final 5 ng/mL), 4 ng/mL BMP4 (final 3 ng/mL), and 8 ng/mL Activin A (R&D, final 4 ng/mL) were added into the well for the AIC. For VCM differentiation, 1.5 mL StemPro-34 medium with the above supplements (without Y-27632 or Matrigel), 10 ng/mL bFGF (final 5 ng/mL), 18 ng/mL BMP4 (final 10 ng/mL), and 12 ng/mL Activin A (final 6 ng/mL) was added to the well to initiate VIC. On day 3, AIC-EBs were washed with IMDM (Thermo Fisher Scientific) once and then transferred to 3 mL StemPro-34 medium containing 2 mM L-glutamine, 50 μg/mL AA, 0.4 mM MTG, 150 μg/mL transferrin, 10 ng/mL VEGF (R&D Systems), 1 μM IWP-3 (Stemgent), 5.4 μM SB431542 (Wako), and 1 μM RA (Wako). VIC-EBs were washed once with IMDM and then transferred to 3 mL StemPro-34 medium with the above supplements (without RA) and 0.6 μM Dorsomorphin (Sigma). On day 6, the medium was transferred to 2 mL StemPro-34 medium containing 2 mM L-glutamine, 50 μg/mL AA, 0.4 mM MTG, 150 μg/mL transferrin, and 5 ng/mL VEGF, and the EBs were maintained in this medium, with changes every 2–3 d until analysis. The plate was incubated at 37 °C in a hypoxia environment (5% O2) for the first 10 d and then transferred to a normoxia environment.
For cardiac differentiation of the 409B2 hiPSC line, the hiPSCs were treated with dissociation solution for human ES/iPS cells (REPROCELL) for 5 min at 37 °C and then suspended at 5 × 104 cells/mL on the same day (0) with the above described medium. The single-cell suspension was seeded at 100 μL/well in a 96-well ultra-low attachment plate (Corning) for EB formation. On day 1, the same medium used above for AIC or VIC was added to each well at 100 μL/well. On day 3, EBs were dissociated using Accumax and then reaggregated with 3 mL StemPro-34 medium containing 2 mM L-glutamine, 50 μg/mL AA, 0.4 mM MTG, 150 μg/mL transferrin, 10 ng/mL VEGF, and 1 μM IWP-3 for VIC. AIC-EBs were washed once with IMDM and then transferred to 3 mL StemPro-34 medium containing 2 mM L-glutamine, 50 μg/mL AA, 0.4 mM MTG, 150 μg/mL transferrin, 10 ng/mL VEGF, 1 μM IWP-3, and 0.5 μM RA. On day 6, EBs were collected in 6-well ultra-low attachment plates and cultured for 4 weeks with the same medium and conditions described above.
Monolayer differentiation into ACMs and VCMs
Monolayer differentiation was performed according to a published protocol37. Briefly, hiPSCs were plated on Matrigel-coated 12-well multi-well plate. Two days after, 6 μM CHIR99021 was added to RPMI supplemented with B27 minus insulin (RPMI-ins, day 0). After 48 h (day 2), the medium was changed to RPMI-ins with 2 μM IWP-1. For AIC, on day3, 1 μM RA was added to the medium for 48 h. The medium was changed to RMPI-ins supplemented with 1 μM RA on day 5, RMPI-ins on day 6, and RPMI supplemented with B27 on day 8. For VIC, the medium was changed to RPMI-ins on day 5 and to RPMI + B27 on day 8. The medium was changed every 2 days during culture. Differentiated cells were used for analyses on days 20–23.
Cell surface marker screening
Day-20 AIC- and VIC-EBs were dissociated using Liberase and Accumax. Dissociated cells were assayed with the Lyoplate Human Cell Surface Marker Screening Panel (BD Biosciences) according to the manufacturer’s instructions. Flow cytometric analysis was performed using FACS Lyric (BD Biosciences).
Flow cytometric analysis
Differentiated EBs were dissociated in the same manner described in the cell surface marker screening section above. For TNNI1-EmGFP reporter hiPSC-CMs, purified mouse anti-human CD151 antibody (BD Biosciences, 1:200) was added as a primary antibody, and CD151 expression was detected using APC-anti-mouse IgG antibody (BD Biosciences, 1:200) or Alexa Fluor 647 goat anti-mouse IgG (Thermo Fisher Scientific, 1:200) as the secondary antibody. 1390C1 and 409B2 hiPSC-CMs were isolated using anti-SIRPa-PE/Cy7 (Biolegend, 1:500) as a cardiomyocyte marker and anti-CD90-APC (BD Biosciences, 1:2500), APC anti-human CD31 (Biolegend, 1:500), AlexaFluor647 anti-CD49a (Biolegend, 1:500), and APC anti-CD140b (Biolegend, 1:500) as non-cardiomyocyte lineage markers24 and directly stained with PE anti-human CD151 (BD Biosciences, 1:200). For Ki-67 staining, fixed cells were stained with anti-human ACTN2 antibody (Creative Diagnostics, 1:200). The cells were then stained with Alexa Fluor 647 anti-mouse/human Ki-67 antibody (BioLegend, 1:400), PE anti-human CD151 (BD Biosciences, 1:200), and Alexa Fluor 488 donkey anti-rabbit IgG (Thermo Fisher Scientific, 1:200) as a secondary antibody against anti-human ACTN2 antibody. For binucleation analysis, dissociated cells were stained with anti-human ACTN2 antibody (Creative Diagnostics, 1:200), Alexa Fluor 488 donkey anti-rabbit IgG (Thermo Fisher Scientific, 1:200) as a secondary antibody, and Hoechst (DOJINDO, 1:1000). The cells were detected and sorted using FACS Aria Fusion (BD Biosciences) and analyzed using FlowJo software (v10.7.1). The gating strategies are shown in Fig. S8.
Immunocytochemistry
AIC- and VIC-CD151high/low CMs were sorted and then seeded at 1 × 105 cells/well on a fibronectin-coated 96-well plate (Corning). The cells were fixed after 5–7 d of culture for 20 min with 4% paraformaldehyde, permeabilized for 15 min with 0.1% TritonX100-PBS, and then blocked for 1 h at room temperature with 2% goat serum/0.1% TritonX100-PBS. The fixed cells were stained using anti-MLC-2A (Synaptic systems, 1:100) and anti-MLC-2V (Proteintech, 1:200) overnight at 4 °C. The following day, the cells were washed twice with PBS and stained using Alexa Fluor 647 goat anti-mouse IgG (Thermo Fisher Scientific, 1:500), Alexa Fluor 647 goat anti-rabbit IgG (Thermo Fisher Scientific, 1:500), or Alexa Fluor 488 goat anti-rabbit IgG (Thermo Fisher Scientific, 1:500) as the secondary antibody for 1 h at 4 °C under dark conditions. The cells were washed twice with PBS and stained with Hoechst (DOJINDO) for 5 min at room temperature. Images were captured using a BZ-X710 (KEYENCE) with a 20× objective.
Gene expression (qPCR)
The total RNA content was prepared using a miRNeasy Micro Kit (QIAGEN), and purified RNA was reverse transcribed into cDNA using a SuperScript™ VILO™ cDNA Synthesis Kit (Thermo Fisher Scientific). Next, qPCR was performed on QuantStudio 7 Flex (Thermo Fisher Scientific) using Taqman probes (Applied Biosystems). The TaqMan probes used are listed (Supplementary Table 2).
Patch-clamp electrophysiology
AIC- and VIC-CD151high/low CMs sorted by flow cytometry were plated on fibronectin-coated cover glasses (3 mm × 7 mm) in a single well of a 24-well plate (2 × 104 cells). The CMs were cultured for 24 h in the same medium used on day 6 of cardiac differentiation described above with 10 μM Y-27632 and maintained in the same medium without Y-27632 with a medium change every 3 d until analysis. The CMs were used for patch-clamp measurements 13–19 d after plating, using an Axopatch 200B amplifier (Molecular Devices) at 5 kHz in the current-clamp mode. APs of spontaneously beating single cells were recorded using the gap-free protocol, and signals were digitized at 10 kHz using Digidata 1322 A. The data were analyzed using pCLAMP 9.2 or 10.7 software (Molecular Devices) and an inverted microscope equipped with differential interface optics (Olympus). The CMs were perfused with Gey’s buffer salt solution (Sigma) and kept at 35–37 °C in the chamber. Patch pipettes were made from glass capillaries using a micropipette puller (P-97/IVF, Sutter Instruments) with tip resistances of 3–5 MΩ when filled with the pipette solution. The pipette solution comprised 130 mM KOH, 20 mM KCl, 1 mM MgCl2, 5 mM NaCl, 130 mM L-Aspartic acid, 10 mM HEPES, 10 mM EGTA, and 5 mM Mg-ATP and was adjusted to pH7.2 with KOH. APs were recorded and analyzed using Clampfit 10.7 software (Axon Instruments), and MDP, APA, APD, and Vmax were calculated based on the average AP of 10 consecutive and stable waves. APD was corrected using Bazett’s correction38.
CD151 KO hiPSC line generation
CD151 KO hiPSCs were generated from the TNNI1-EmGFP hiPSC line utilizing the CRISPR-Cas9 system. Gene editing was performed according to a published protocol39, using a gRNA (target sequence: 5’-CAGGTTCCGACGCTCCTTGA-3’). Briefly, to form gRNA, an equimolar amount of crRNA and tracrRNA (IDT) was hybridized for 5 min at 95 °C and then cooled to 20 °C using a thermal cycler with a ramp rate setting of −0.1 °C/s. RNP complexes were formed with 61 pmol of gRNA and Cas9 nuclease each and transfected to TNNI1-EmGFP hiPSCs by electroporation. Transfected hiPSCs were cultured and analyzed by flow cytometry. CD151 KO hiPSCs were purified by sorting of CD151-negative populations. To validate them at the genome level, genomic DNA was isolated from TNNI1-EmGFP hiPSCs and CD151 KO hiPSCs. We then sequenced the regions around the target site of CD151 and five off-target genes predicted by CRISPOR40 using primers listed in Supplementary Table 3. The Sanger sequencing results were analyzed to identify the constitution of indels and the gene-editing efficiency using DECODR (https://decodr.org/).
Western blots for CD151 expression
Proteins were extracted from cells with RIPA buffer, with protein concentrations measured using the Pierce BCA Protein Assay Kit. Proteins were separated by SDS-PAGE using Criterion TGX Precast Gel (BioRad) and transferred to PVDF membrane. CD151 monoclonal antibody (2A8G8) (Thermo Fisher SCIENTIFIC, 1:2000) and anti-Actin monoclonal antibody (MAB1501) (Merck Millipore, 1:5000) were used as primary antibodies. ECL peroxidase-labeled anti-mouse antibody (NA931) (GE Healthcare, 1:5000) was used as the secondary antibody.
Optical recording of APs with 4-AP and CCh
AIC-CMs and LY411575/CD151low ACMs were sorted on day 20 and plated as 5 μL cell suspensions (5 × 104 cells) on a fibronectin-coated glass-bottom dish to form CM sheets, with the medium added 2 h later. The medium was changed every 2–3 d during CM culture. The CMs were treated with FluoVolt dye (Thermo Fisher Scientific) following the manufacturer’s instructions. One hour after setting the cells in a stage top incubator (TOKAI HIT) at 37 °C (5% CO2, 95% air), fluorescence measurements were acquired with an ECLIPSE Ti-E inverted fluorescence microscope (Nikon), objective Fluor 10×/0.45 (Nikon), X-Cite TURBO (Excelitas Technologies), EM-CCD camera ImagEM (Hamamatsu Photonics), and AQUACOSMOS 2.6 software (Hamamatsu Photonics) that set the excitation wavelength at 490 nm and bandwidth at 10 nm. The X-Cite TURBO was set at 5% output. CMs cultured for 7 d were used to record the subarray images every 5 ms. Binning was set to 1 × 1. The regions of interest (ROIs) were defined as whole 512 × 64 pixels in the monolayers. The CMs were paced at 4 Hz with 2–5 ms depolarizing pulses at 10 V using a pulse stimulator (Master-9, A.M.P.I., Jerusalem, Israel) with an interelectrode distance of 12 mm (Intermedical, Osaka, Japan). The effects of drugs were measured 10 min after exposure to 50 μM 4-AP. Waveform traces were superimposed using OriginPro 2021 (OriginLab, Northampton, MA, USA). 4-AP (Sigma) was prepared as a 50 mM stock solution in Gey’s buffer salt solution, with pH adjusted to 7.4, and stored at −20 °C. CCh (Sigma) was prepared as a 10 mM stock solution in Gey’s buffer salt solution and stored at −20 °C.
Library preparation and RNA-seq
Total RNA was prepared with a miRNeasy Micro Kit. RNA-seq libraries were generated using TruSeq Stranded Total RNA (Illumina) according to the manufacturer’s instructions. RNA-seq libraries were sequenced on a NextSeq 500 using High Output Kit v2.0 with single-end reads and 75 cycles (Illumina). RNA-seq reads were mapped to the human reference genome (hg38) using STAR41 and normalized using DESeq242. Genes with less than 10 normalized counts in an average of 12 samples were excluded, and 17142 genes were finally included in the data sets for the analysis. DEGs were identified using Wald’s test in DESeq2. P values of the genes were adjusted via the Benjamini-Hochberg procedure, and adjusted p values < 0.05 were considered significant. PCA was performed using the prcomp package in R (v4.0.3) with the normalized gene expression data. GO and Reactome pathway enrichment analyses were performed using the geneXplain® platform (geneXplain) for DEGs. Hierarchical clustering was performed using Spearman’s distance and Ward’s algorithm, and heatmaps were generated using heatmap.2 in gplots (v3.1.1) R package.
Single-cell RNA sequence analysis
To assess the tissue-specific expression patterns of CD151 in the developing human and mouse heart, we utilized publicly available single-cell RNA sequencing (scRNAseq) datasets30,31 (Human: GSE106118, Mouse: GSE193346) across distinct developmental stages. For human samples, transcriptomic data from left atrial (LA) and ventricular (LV) tissues at 7, 13, and 20 gestational weeks were analyzed. Violin plots were generated at each stage to compare expression profiles, and statistical significance was evaluated using the Mann–Whitney U test. A parallel methodology was applied to mouse samples, focusing on embryonic days 10.5 (E10.5) and 18 (E18), and postnatal day 5 (P5) with corresponding statistical analysis.
Notch inhibitor treatment
EBs were treated with 3 μM LY411575 (Selleck Chemicals), a gamma-secretase inhibitor, from days 8–20. On day 20, the EBs were dissociated and sorted in the same way as explained above in the flow cytometric analysis section.
Statistics and Reproducibility
GraphPad Prism (Graph Pad Software Inc.) was used to perform statistical analyses and create graphs and bar plots. Quantitative data are shown as the mean ± standard error of the mean (SEM), as indicated in the figure legends. Sample sizes (n) used in each experiment are indicated in the figure legends. Statistical significance was determined using Student’s t test (unpaired or paired, two-tailed) and one-way analysis of variance (ANOVA) followed by Tukey’s honest significant difference (HSD) test or Dunnett’s test, unless specified otherwise.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Supplementary information
Acknowledgements
This work was supported by the Takeda-CiRA collaboration program and the following grants: JSPS KAKENHI Grants (JP18K15120, JP18KK0461, JP19K16041, JP17H04176, and JP 21H02912); the Leducq Foundation (18CVD05); the Research Center Network for Realization of Regenerative Medicine, AMED (JP20bm0104001, JP21bm0204003, JP21bm0804008, and JP21bm0804022); the Acceleration Program of R&D and implementation for Regenerative Medicine and Cell and Gene Therapy, AMED (JP23bm1423011 and JP23bm132300); the Research on Regulatory Science of Pharmaceuticals and Medical Devices, AMED (JP21mk0101189 and JP22mk0101241); the Translational Research grant, AMED (JP22ym0126091); the Research Project for Practical Applications of Regenerative Medicine, AMED (JP21bk0104095); and the iPS Cell Research Fund. We thank Shinya Yamanaka, Seigo Izumo, Yasushi Kajii, and Steve Okada for supporting this project; Takako Sono, Ayaka Sakoda, and Izumi Yamada (T-CiRA) for technical assistance; Kaoru Shimizu, Rumi Fujihara, and Mikako Marx-Mori (CiRA), Takanori Matsuo, Aya Higashide, and Yumi Monnai (T-CiRA) for their administrative support; and Peter Karagiannis and Kelvin Hui (CiRA) for critical reading of the manuscript. In preparing this work, ChatGPT was used to proofread the manuscript.
Author contributions
M.N.K., K.M., A.H., K.I., T.N. and Y.Y. conceived and designed the study. M.N.K., Y.N., M.S., T.W., Misato.N., K.C. and S.C.N. performed the experimental work. M.N.K., M.S., C.O., Megumi. N. and R.H. performed and analyzed the RNA-seq experiments. M.N.K., K.M. and Y.Y. interpreted the data and wrote the manuscript. All authors discussed the results.
Peer review
Peer review information
Communications Biology thanks Kyle Loh, Lukas Cyganek, Haodi Wu, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editors: Ngan Huang, Eve Rogers and Christina Karlsson Rosenthal. A peer review file is available.
Data availability
The RNA-seq data reported in this paper have been deposited in NCBI’s Gene Expression Omnibus and are accessible through GEO series accession number GSE179769. Source data are provided in Supplementary Data 3. The data that support the findings of this study are available from the corresponding author upon reasonable request.
Competing interests
The authors declare the following competing interests: Y.Y. is a scientific advisor of Orizuru Therapeutics, Inc. and received research funding from Takeda Pharmaceutical Company, Ltd. and Altos Labs, Inc. S.C.N. and K.I. are employees of Takeda Pharmaceutical Company, Ltd. T.N. is an employee of Orizuru Therapeutics, Inc. M.N.K, K.M., and Y.Y. are the inventors of the patent application (WO2021/033699 and 2022/125789). The other authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Kenji Miki, Email: kenjimiki.prime@osaka-u.ac.jp.
Yoshinori Yoshida, Email: yoshinor@cira.kyoto-u.ac.jp.
Supplementary information
The online version contains supplementary material available at 10.1038/s42003-024-05809-2.
References
- 1.Zhang J, et al. Functional cardiomyocytes derived from human induced pluripotent stem cells. Circ. Res. 2009;104:e30–e41. doi: 10.1161/CIRCRESAHA.108.192237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ma J, et al. High purity human-induced pluripotent stem cell-derived cardiomyocytes: electrophysiological properties of action potentials and ionic currents. Am. J. Physiol. Heart Circ. Physiol. 2011;301:H2006–H2017. doi: 10.1152/ajpheart.00694.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burridge PW, et al. Chemically defined generation of human cardiomyocytes. Nat. Methods. 2014;11:855–860. doi: 10.1038/nmeth.2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang Q, et al. Direct differentiation of atrial and ventricular myocytes from human embryonic stem cells by alternating retinoid signals. Cell Res. 2011;21:579–587. doi: 10.1038/cr.2010.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Devalla HD, et al. Atrial-like cardiomyocytes from human pluripotent stem cells are a robust preclinical model for assessing atrial-selective pharmacology. EMBO Mol. Med. 2015;7:394–410. doi: 10.15252/emmm.201404757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee JH, Protze SI, Laksman Z, Backx PH, Keller GM. Human pluripotent stem cell-derived atrial and ventricular cardiomyocytes develop from distinct mesoderm populations. Cell Stem Cell. 2017;21:179–194.e174. doi: 10.1016/j.stem.2017.07.003. [DOI] [PubMed] [Google Scholar]
- 7.Marvin MJ, Di Rocco G, Gardiner A, Bush SM, Lassar AB. Inhibition of Wnt activity induces heart formation from posterior mesoderm. Genes Dev. 2001;15:316–327. doi: 10.1101/gad.855501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schneider VA, Mercola M. Wnt antagonism initiates cardiogenesis in Xenopus laevis. Genes Dev. 2001;15:304–315. doi: 10.1101/gad.855601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Atsuhiko T, et al. Developmental stage-specific biphasic roles of Wnt/β-catenin signaling in cardiomyogenesis and hematopoiesis. Proc. Natl. Acad. Sci. 2006;103:19812–19817. doi: 10.1073/pnas.0605768103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ueno S, et al. Murry Biphasic role for Wnt/β-catenin signaling in cardiac specification in zebrafish and embryonic stem cells. Proc. Natl. Acad. Sci. 2007;104:9685–9690. doi: 10.1073/pnas.0702859104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marques SR, Yelon D. Differential requirement for BMP signaling in atrial and ventricular lineages establishes cardiac chamber proportionality. Dev. Biol. 2009;328:472–482. doi: 10.1016/j.ydbio.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Keegan BR, Meyer D, Yelon D. Organization of cardiac chamber progenitors in the zebrafish blastula. Development. 2004;131:3081–3091. doi: 10.1242/dev.01185. [DOI] [PubMed] [Google Scholar]
- 13.Yao Y, Marra AN, Yelon D. Pathways regulating establishment and maintenance of cardiac chamber identity in Zebrafish. J. Cardiovasc. Dev. Dis. 2021;8:13. doi: 10.3390/jcdd8020013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Niederreither K, et al. Embryonic retinoic acid synthesis is essential for heart morphogenesis in the mouse. Development. 2001;128:1019–1031. doi: 10.1242/dev.128.7.1019. [DOI] [PubMed] [Google Scholar]
- 15.Hochgreb T, et al. A caudorostral wave of RALDH2 conveys anteroposterior information to the cardiac field. Development. 2003;130:5363–5374. doi: 10.1242/dev.00750. [DOI] [PubMed] [Google Scholar]
- 16.Ryckebusch L, et al. Retinoic acid deficiency alters second heart field formation. Proc. Natl. Acad. Sci. 2008;105:2913–2918. doi: 10.1073/pnas.0712344105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu SP, et al. Atrial identity is determined by a COUP-TFII regulatory network. Dev. Cell. 2013;25:417–426. doi: 10.1016/j.devcel.2013.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kattman SJ, et al. Stage-specific optimization of activin/nodal and BMP signaling promotes cardiac differentiation of mouse and human pluripotent stem cell lines. Cell Stem Cell. 2011;8:228–240. doi: 10.1016/j.stem.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 19.Bizy A, et al. Myosin light chain 2-based selection of human iPSC-derived early ventricular cardiac myocytes. Stem Cell Res. 2013;11:1335–1347. doi: 10.1016/j.scr.2013.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen Z, et al. Subtype-specific promoter-driven action potential imaging for precise disease modelling and drug testing in hiPSC-derived cardiomyocytes. Eur. Heart J. 2017;38:292–301. doi: 10.1093/eurheartj/ehw189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang JZ, et al. A human iPSC double-reporter system enables purification of cardiac lineage subpopulations with distinct function and drug response profiles. Cell Stem Cell. 2019;24:802–811.e805. doi: 10.1016/j.stem.2019.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Veevers J, et al. Cell-surface marker signature for enrichment of ventricular cardiomyocytes derived from human embryonic stem cells. Stem Cell Rep. 2018;11:828–841. doi: 10.1016/j.stemcr.2018.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miki K, et al. ERRγ enhances cardiac maturation with T-tubule formation in human iPSC-derived cardiomyocytes. Nat. Commun. 2021;12:3596. doi: 10.1038/s41467-021-23816-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dubois NC, et al. SIRPA is a specific cell-surface marker for isolating cardiomyocytes derived from human pluripotent stem cells. Nat. Biotechnol. 2011;29:1011–1018. doi: 10.1038/nbt.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koibuchi N, Chin MT. CHF1/Hey2 plays a pivotal role in left ventricular maturation through suppression of ectopic atrial gene expression. Circ. Res. 2007;100:850–855. doi: 10.1161/01.RES.0000261693.13269.bf. [DOI] [PubMed] [Google Scholar]
- 26.Churko JM, et al. Defining human cardiac transcription factor hierarchies using integrated single-cell heterogeneity analysis. Nat. Commun. 2018;9:4906. doi: 10.1038/s41467-018-07333-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Derks W, Bergmann O. Polyploidy in cardiomyocytes: roadblock to heart regeneration? Circ. Res. 2020;126:552–565. doi: 10.1161/CIRCRESAHA.119.315408. [DOI] [PubMed] [Google Scholar]
- 28.Bergmann O, et al. Dynamics of cell generation and turnover in the human heart. Cell. 2015;161:1566–1575. doi: 10.1016/j.cell.2015.05.026. [DOI] [PubMed] [Google Scholar]
- 29.Termini CM, Gillette JM. Tetraspanins function as regulators of cellular signaling. Front. Cell Dev. Biol. 2017;5:34. doi: 10.3389/fcell.2017.00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Feng W, et al. Single-cell transcriptomic analysis identifies murine heart molecular features at embryonic and neonatal stages. Nat. Commun. 2022;13:7960. doi: 10.1038/s41467-022-35691-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cui Y, et al. Single-cell transcriptome analysis maps the developmental track of the human heart. Cell Rep. 2019;26:1934–1950.e1935. doi: 10.1016/j.celrep.2019.01.079. [DOI] [PubMed] [Google Scholar]
- 32.Wiencierz AM, et al. Differential expression levels of integrin alpha6 enable the selective identification and isolation of atrial and ventricular cardiomyocytes. PLoS One. 2015;10:e0143538. doi: 10.1371/journal.pone.0143538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goversen B, van der Heyden MAG, van Veen TAB, de Boer TP. The immature electrophysiological phenotype of iPSC-CMs still hampers in vitro drug screening: special focus on IK1. Pharm. Ther. 2018;183:127–136. doi: 10.1016/j.pharmthera.2017.10.001. [DOI] [PubMed] [Google Scholar]
- 34.Evan, S. B. et al. Notch signaling commits mesoderm to the cardiac lineage. bioRxiv, 2020.2002.2020.958348 (2020).
- 35.Raulf A, et al. Transgenic systems for unequivocal identification of cardiac myocyte nuclei and analysis of cardiomyocyte cell cycle status. Basic Res. Cardiol. 2015;110:33. doi: 10.1007/s00395-015-0489-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miki K, et al. Efficient detection and purification of cell populations using synthetic MicroRNA switches. Cell Stem Cell. 2015;16:699–711. doi: 10.1016/j.stem.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 37.Kleinsorge M, Cyganek L. Subtype-directed differentiation of human iPSCs into atrial and ventricular cardiomyocytes. STAR Protoc. 2020;1:100026. doi: 10.1016/j.xpro.2020.100026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.BAZETT HC. An analysis of the time-relations of electrocardiograms. Ann. Noninvasive Electrocardiol. 1997;2:177–194. doi: 10.1111/j.1542-474X.1997.tb00325.x. [DOI] [Google Scholar]
- 39.Maurissen TL, Woltjen K. Synergistic gene editing in human iPS cells via cell cycle and DNA repair modulation. Nat. Commun. 2020;11:2876. doi: 10.1038/s41467-020-16643-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Concordet JP, Haeussler M. CRISPOR: intuitive guide selection for CRISPR/Cas9 genome editing experiments and screens. Nucleic Acids Res. 2018;46:W242–W245. doi: 10.1093/nar/gky354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dobin A, et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 2013;29:15–21. doi: 10.1093/bioinformatics/bts635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The RNA-seq data reported in this paper have been deposited in NCBI’s Gene Expression Omnibus and are accessible through GEO series accession number GSE179769. Source data are provided in Supplementary Data 3. The data that support the findings of this study are available from the corresponding author upon reasonable request.