Abstract
Auxin-induced callus formation was largely dependent on the function of Lateral Organ Boundaries Domain (LBD) family transcription factors. We previously revealed that two IGMT (Indole glucosinolate oxy-methyl transferase) genes, IGMT2 and IGMT3, may be involved in the callus formation process as potential target genes of LBD29. Overexpression of the IGMT genes induces spontaneous callus formation. However, the details of the IGMT involvement in callus formation process were not well studied. IGMT1-4, but not IGMT5, are targeted and induced by LBD29 during the early stage of callus formation. Cell membrane and nucleus localized IGMT3 was mainly expressed in the elongation and maturation zones tissues of the primary root and lateral root, which could be further accumulated after CIM treatment. The igmts quadruple mutant, which obtained by CRISPR/Cas9 technology, exhibits a phenotype of attenuated callus formation. Enhanced indole glucosinolate anabolic pathway caused by IGMT1-4 overexpression promotes callus formation. In addition, the IGMT genes were involved in the reactive oxygen species homeostasis, which could be responsible for its role on callus formation. This study provides novel insights into the role of IGMTs gene-mediated callus formation. Activation of the Indole glucosinolate anabolic pathway is an inducing factor for plant callus initiation.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12298-023-01409-2.
Keywords: IGMT (Indole glucosinolate oxy-methyl transferase), Callus formation, Arabidopsis thaliana, ROS homeostasis
Introduction
Plant cells have long and widely been regarded as totipotent (Reinert and Backs 1969), that is, the differentiated tissues can be induced to form pluripotent cell mass termed callus in response to different stimuli (Sugiyama 2018). Plant callus formation events can be divided into two main categories based on their induction factors (Ikeuchi et al. 2013; Xu and Hu 2020): hormone-induced callus formation, which is mainly dependent on appropriate concentrations of two phytohormones, auxin and cytokinin (Skoog and Miller 1957); and wounding-induced callus formation, which is the active defense responses for plants to repair themselves under wounding stress (Ikeuchi et al. 2013; Sugimoto et al. 2011).
It was demonstrated that the hormone-induced callus formation process shares the pathway with plant lateral root initiation (Sugimoto et al. 2010). Importantly, auxin-induced callus formation is the initial step during in vitro regeneration of plants, in which pericycle or pericycle-like cells in explant tissues are reprogrammed into the pluripotent cells for subsequent regeneration of root and shoot (Shang et al. 2016). The IAA14-Auxin Response Factor 7/19 (ARF7/19)-Lateral Organ Boundaries Domains (LBDs) signaling module plays a critical role in this process (Fan et al. 2012).
Four Arabidopsis Lateral Organ Boundaries Domain (LBD) transcription factors, LBD16, LBD17, LBD18, and LBD29 are proved to be the key regulators to trigger auxin-induced callus formation by interacting with Basic Leucine Zipper 59 (bZIP59) (Xu et al. 2018). Further, the bZIP59–LBD complex could directly activate the expression of FAD-Binding Berberine (FAD-BD) to trigger callus formation (Xu et al. 2018). During lateral root development, LBD18 and LBD29 directly regulates cell wall-loosening factor EXPANSIN 14 (EXP14) and auxin influx carrier LAX3, respectively, in promoting lateral root emergence (Lee et al. 2012; Porco et al. 2016), and LBD18/LBD33 promotes cell proliferation in lateral root organogenesis by activating the expression of cell cycle regulator E2Fa (Berckmans et al. 2011). In our previous work, we identified the candidate LBD29-targeted genes during the early stage of callus formation were mostly involved in the regulation of methylation, reactive oxygen species (ROS) and lipid metabolism, cell wall hydrolysis, and repression of light response and/or photosynthesis by chromatin immunoprecipitation-based sequencing (ChIP-seq) and RNA-seq, suggesting that the alteration of these biological processes were important molecular events behind LBD-induced cell reprogramming. However, most of the LBD-targeted genes during callus formation were not well investigated at present.
The Indole glucosinolate oxy-methyl transferase (IGMT) gene family encodes a class of O-methyltransferase (OMT) family proteins (Pfalz et al. 2009). There are five IGMT homolog genes in arabidopsis, of which IGMT1-4, share more than 94% identity in amino acids sequence, were arranged on the left arm of chromosome 1 tandemly, while IGMT5, encodes a protein that exhibits approximately 70% amino acid sequence similarity to IGMT1-4, localized alone on the right arm of chromosome 1 (Pfalz et al. 2016). IGMT1-2 and possibly IGMT3-4 play the role of converting 4-hydroxyindol-3-ylmethyl glucosinolate (4OHI3M) to 4-methoxyindol-3-ylmethyl glucosinolate (4MOI3M) (Pfalz et al. 2011), while IGMT5 converts 1-hydroxyindol-3-ylmethyl glucosinolate (1OHI3M) to 1-methoxyindol-3-ylmethyl glucosinolate (1MOI3M) (Pfalz et al. 2016).
Previous studies have mainly focused on the role of IGMT and its related metabolites in plants defending against pests and pathogens (Bednarek et al. 2009; Chhajed et al. 2020; Halkier and Gershenzon 2006; Pedras et al. 2011). Indole glucosinolates (IGS) and their breakdown products play an important role in host recognition during ovipositing and feeding of pests such as diamondback moth (Plutella xylostella) (Sun et al. 2009), Pieris rapae (de Vos et al. 2008) and green peach aphid (Myzus persicae) (Kim and Jander 2007). In addition, IGS have been shown to enhance the innate immunity of plants (Clay et al. 2009), allowing them to acquire broad-spectrum resistance to many fungal pathogens (Bednarek et al. 2009), such as Alternaria brassicicola (Tao et al. 2022), Pseudomonas (Fan et al. 2011) and Pectobacterium brasiliense (P. brasiliense) (Yi et al. 2022). The expression of IGMT genes could be induced by a variety of biotic and abiotic stress signals such as Yariv phenylglycoside triggered wound-like responses (Guan and Nothnagel 2004), γ-Irradiation (Nagata et al. 2005), pathogen Botrytis cinerea (Xu et al. 2016) and Myzus persicae feeding (Kim and Jander 2007), but could be repressed by sucrose (Rogers et al. 2005). In contrast to the in-depth study of the function of IGMT genes and their metabolites in affecting plant immunity, the understanding of their regulation of plant growth and development has been just beginning.
Reactive oxygen species (ROS), a category of oxygen-containing reactive compounds, which include the superoxide species (·O2−), hydrogen peroxide (H2O2), and hydroxyl radical (·OH), etc. are produced in all living organisms as a consequence of aerobic metabolism. Traditionally, ROS have thought to be involved in the response of plants and animals to different biotic and abiotic stresses (Castro et al. 2021; Finkel and Holbrook 2000; Mittler et al. 2022). However, more and more studies have shown that ROS plays an important role in plant and animal stem cell fate determination in recent years (Jang and Sharkis 2007; Ludin et al. 2014; Zeng et al. 2017).
To investigate the downstream target genes of LBD29 in callus formation, we generated transgenic ProXVE:LBD29 plants with a β-estradiol inducible promoter and analyzed genome-wide transcriptome profiling by RNA-seq in a previous study (Xu et al. 2017). By comparing the gene expression profiles between the control group treated by β-estradiol for 0 h and those treated for 2, 4, and 8 h, the IGMT family containing five members, four of which IGMT1, IGMT2, IGMT3, and IGMT4 were found to be activated by LBD29 rapidly and significantly (Xu et al. 2017). Yeast one-hybrid and chromatin immunoprecipitation (CHIP) assays demonstrated LBD29 could bind to the promoter of IGMT2 and IGMT3 directly (Xu et al. 2017). Compared with control seedlings, T1 transgenic plants overexpressing IGMT2 or IGMT3 exhibited different degrees of callus spontaneously when cultured on medium without phytohormones (Xu et al. 2017). In this research, we further confirmed that the role of IGMT in plant callus formation is related to its metabolites IGS, and the disturbance of IGMTs’ function may influence the ROS homeostasis in vivo.
Materials and methods
Plant materials and growth conditions
Arabidopsis thaliana ecotype Columbia-0 (Col-0) was used in this study. Seeds were germinated and cultured on 1/2 MS medium (1/2 MS salts, 1% sucrose, 0.6% agar) at 22 ± 1℃ under 16 h light/8 h dark. For callus induction, 7-day-old seedlings were transferred to callus-inducing medium (CIM) with different concentrations of 2,4-D (B5 salts, 0.6% phytogel, 0.05 µg/ml kinetin, 0.05, 0.1, 0.2, 0.5 µg/ml 2,4-D) for the times indicated. T-DNA insertion mutant igmt3 (CS829786) was obtained from the ABRC, and then cultured on CIM with varied concentrations of 2,4-D to observe the callus formation phenotype. For phenotype observation, T1 generation seeds were sown on 1/2 MS medium containing 50 mg/L kanamycin and 100 mg/L carbenicillin and cultured for 7 days. The obtained T-DNA insertion plants were transferred to B5 medium containing 100 mg/L carbenicillin for 20 days, and the callus formation phenotype was observed.
Quantitative RT-PCR
Whole seedlings were used as materials to extract total RNA using RNeasy plant mini kit (Qiagen). The Super-Script III first-strand cDNA synthesis system (Invitrogen) was used for cDNA synthesis. The quantitative RT-PCR was performed with SYBR Green PCR Master Mix on Roche LightCycler 96. Three biological replicates were settled for each treatment. ACTIN2 was used as a reference to normalize the mRNA levels. Gene-specific primers used for qRT-PCR are listed in Supplementary Table S1.
Plasmid construction and transformation
To construct the ProIGMT2:GUS, ProIGMT3:GUS vectors, approximately 2000 bp genomic DNA sequence of IGMT2, IGMT3 promoter were amplified by PCR and cloned into the pCAMBIA 1300 vector with the GUS sequence. Pro35S:IGMT3, Pro35S:IGMT2 transgenic plants were described previously (Xu et al. 2017). To construct the p35S:MYB122, and p35S:UBP1, the cDNAs of MYB122 and UBP1 were cloned into the pVIP96 vector, respectively. To create the pIGMT3:IGMT3-GFP, 2000 bp DNA sequence of IGMT3 promoter and CDS were amplified by PCR, then both cloned into pMDC83 with GFP coding sequence using Universal One Step Cloning Kit (YEASEN). For generation of igmt1 igmt2 igmt3 igmt4 quadruple mutants by CRISPR/Cas9 genome editing technology, we screened two sgRNA targets on http://www.genome.arizona.edu/crispr/CRISPRsearch.html, and design the primers. The pCBC-DT1T2 was used as the template for PCR amplification with the four primers, and cloned into pHEE401 vector. All primers used for the generation of the constructs are listed in Supplementary Table S1. For plant transformation, all these constructs were introduced into Agrobacterium tumefaciens strain ABI or EHA105, and the Agrobacterium was transformed into Arabidopsis Col-0 by the floral dip method (Clough and Bent 1998).
GUS staining
GUS activity of ProIGMT3:GUS and ProIGMT2:GUS was performed by incubating the transgenic seedlings in 90% acetone for 20 min and then transferred to GUS solution (1 M Phosphate buffer (PH 7.2), 10% Triton X-100, 100 mM Potassium ferricyanide (K3Fe(CN)6), 100 mM Potassium ferrocyanide (K4Fe(CN)6), 100 mM X-gluc) for 0.5 or 1 h at 37 °C. Finally, the samples were rinsed with 70% ethanol and analyzed under the microscope (Olympus).
Confocal microscopy
To visualize the sub-cellular localization of IGMT3-GFP, a drop of FM4-64 was added to the transgenic pIGMT3:IGMT3-GFP seedlings, which were observed under a Leica SP5 confocal microscope. GFP was excited by an argon laser at 488 nm, and the emission spectra were collected between 500 and 550 nm. The FM4-64 signal was detected by excitation with an argon laser at 488 nm and a spectral detector set at 640 nm for the emission.
Phylogenetic analyses
The gene tree was built by MEGA6 with the full-length amino acid sequences of IGMT1-5 in Arabidopsis, which download from TAIR (https://www.arabidopsis.org/), using the neighbor-joining (NJ) method (Tamura et al. 2013).
NBT staining assay
7-day-old WT, igmt1 igmt2 igmt3 igmt4 mutant, transgenic Pro35S:IGMT3 seedlings incubated on CIM for 0 d or 4 d were stained in a NBT staining solution (1 mg/mL NBT, 50 mM potassium dihydrogen phosphate buffer, pH 7.6) in dark at room temperature for 10–30 min and transferred to the boiling destaining solution (ethanol: glycerin: glacial acetic acid = 3:1:1) for 10 min. The samples were fixed and imaged under the microscope (Olympus).
DMTU treatment
For DMTU treatment, 5-day-old WT, igmt1 igmt2 igmt3 igmt4 mutant, transgenic Pro35S:IGMT3 seedlings were transferred from 1/2 MS medium to CIM with DMSO or 10 mM DMTU for 7 days.
Accession numbers
The sequence data for the genes in this article can be found in Arabidopsis Genome Initiative as the following accession numbers: IGMT1 (At1g21100), IGMT2 (At1g21120), IGMT3 (At1g21110), IGMT4 (At1g21130), AOX1A (At3g22370), AOX1C (At3g27620), AOX1D (At1g32350), AOX2 (At5g64210), PRX52 (At5g05340), PER58 (At5g19880), PER15 (At2g18150), PER49 (At4g36430) and ACTIN2 (At3g18780).
Results
Temporal and spatial expression of IGMTs during callus formation
To explore whether IGMT1, IGMT2, IGMT3, and IGMT4 function in callus formation, we first analyzed their expression levels in Arabidopsis transferred to callus induction medium (CIM) at different times. As shown in Figure S1, all four genes were induced in the early stage of callus induction, among which IGMT3 exhibited a slightly higher expression level than others, whereas the expressions were decreased after 8 h treatment. This result confirmed that LBD29-targeted IGMT genes are induced obviously during the early stage of callus formation.
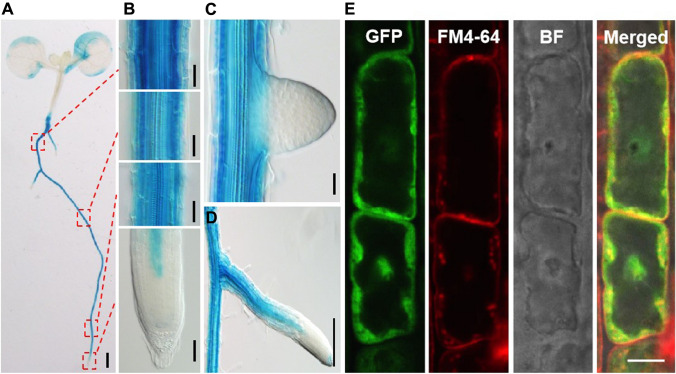
To further reveal the spatial and temporal changes of IGMT expression during callus formation, we generated ProIGMT2:GUS and ProIGMT3:GUS transgenic plants and analyzed their expression patterns during callus formation. As shown in Fig. 1A–D, IGMT3 in seedlings without CIM treatment was mainly expressed in the elongation and maturation zone tissues of the primary root and lateral root, including exodermis, cortex, endodermis, pericycle, and vascular, but not in dividing cells. The GUS staining was stronger in the cortex than other cell layers in the maturation zone and stronger in the vascular of the elongation zone. Meanwhile, we analyzed the subcellular localization of IGMT3, as shown in Fig. 1E, IGMT3 protein was localized in the cell membrane and cell nucleus.
Fig. 1.
Expression and subcellular localization of IGMT3. A ProIGMT3:GUS reporter expression was detected in mature and expanding root tissues. B Enlarged images of root segments in (A). From top to bottom, the four images represent the mature zone, the mature zone, the elongation zone and the meristem zone. C The reporter was not expressed in the newly emerged lateral root but expressed in the mature lateral root (D). (E) IGMT3 protein was localized in the cell membrane and nucleus. The Scale bars for Figure A, D and E are 1 mm, 1 mm and 100 μm, respectively. And the Scale bar for Figure B and C is 100 μm
After the transgenic plants were transferred to the CIM for 1–4 h, the GUS staining continued to increase, and gradually decreased after 4 h. After the seedlings were incubated on CIM for 48 h, the expression of IGMT3 was reduced in dividing cells of formed callus obviously (Fig. 2A). In the same way, we observed the expression of IGMT2 in the transgenic plant ProIGMT2:GUS, the GUS activity was similar to IGMT3, which increased at the early stage of callus induction and gradually decreased after 8 h of induction (Fig. 2B). These results further suggested that IGMT genes have probably been involved in the early process of callus initiation, but not in the late process of callus formation and maintenance.
Fig. 2.
Spatiotemporal expression of IGMTs during callus formation. A 7-day-old ProIGMT3:GUS and B ProIGMT2:GUS transgenic seedlings incubated on CIM for 0 h, 1 h, 2 h, 4 h, 8 h, 12 h, 24 h, and 48 h were assayed for GUS staining for 0.5 h. Insets show the boxed area of the mature zone. Scale bars = 2 mm (seedlings)/100 µm (enlarged images)
Repression of IGMT function by CRISPR/Cas9 attenuates callus formation
Although previous studies have shown that overexpression of IGMT genes can induce spontaneous callus formation (Xu et al. 2017), genetic evidence is insufficient. To further investigate the role of IGMT in callus formation, a T-DNA insertion mutant, in which no full-length transcript of IGMT3 could be detected, was obtained from the ABRC, yet it did not exhibit any defect in callus formation even under auxin treatment conditions (Figure S2).
There are five members of the IGMT family in Arabidopsis, namely IGMT1-5, among which IGMT1-4 and IGMT5 are located in two adjacent branches of the oxy-methyltransferase cluster analysis (Fig. 3A). Coincidentally, the expression of IGMT1-4, but not IGMT5, was induced by LBD29 (Xu et al. 2017). The protein structure of IGMT is conserved and consists of two functional domains, the N-terminal protein-protein interaction or substrate binding domain contains 55 amino acids and the C-terminal AdoMet binding domain with 218 amino acids. Considering that IGMT1-4 are coupled on the chromosome and have similar nucleotide sequences, along with probably the functional redundancy of these genes, CRISPR/Cas9 technology was used to generate the igmt1 igmt2 igmt3 igmt4 (igmts) quadruple mutants. After careful selection without off-target effect, the consistent nucleotide sequence (GATAACGAGTTGGGTTTGATGG) with protospacer adjacent motif (PAM) site in the first exon region of IGMT1-4 was selected as the target site (Fig. 3B). After resistance screening and sequencing analysis, three homozygous igmt quadruple mutants with different mutation forms were finally obtained in T3 transgenic plants (Fig. 3C). In igmt1 igmt2 igmt3 igmt4-1 (igmts-1) mutant, seven nucleotides were deleted in the target site of IGMT1 resulting in premature termination of protein translation, which changes from 373 amino acids to 24 amino acids. One nucleotide addition occurred in IGMT2, IGMT3, and IGMT4, leading to protein changes from 373 amino acids to 42, 42, and 51 amino acids, respectively. Similarly, in igmt1 igmt2 igmt3 igmt4-2 (igmts-2) mutant, the mutation in IGMT1-4 caused the protein to turn into 51, 78, 26, and 29 amino acids, respectively, in igmt1 igmt2 igmt3 igmt4-3 (igmts-3) mutant; IGMT1-4 protein mutated into 51, 42, 26, and 29 amino acids, respectively. Therefore, in these three mutant lines, the methylation domain was completely lost, and the interaction domain was incomplete.
Fig. 3.
The igmts exhibits defect in callus formation. A Phylogenetic analysis of IGMTs in Arabidopsis. B Schematic diagram of the protein structure of IGMT and the sequence of the targeted site. The green box represents the substrate binding domain, the red box represents the AdoMet binding domain, and the green number and the red number indicate the site of each domain. C The mutations at IGMT1, IGMT2, IGMT3 and IGMT4 locus in three lines of igmts. Mutations are marked in red colour, the number indicates the amino acids of each protein in three igmt lines. D The callus-forming phenotype of 7-day-old WT and igmts seedlings cultured on CIM for 5 days. E Quantification of the callus initiation on the primary roots of seedlings cultured on CIM for 4 days. Scale bar = 10 mm. Error bars = ± SD, n > 12 (Color figure online)
Then the callus induction experiments were carried out with the three igmt homozygous lines and wild-type plants. It was found that callus formation of igmt mutants was obviously weakened when treated with CIM medium containing different concentrations of 2,4-D compared with those of wild-type plants (Fig. 3D, E). These results indicate that IGMT plays an important role during callus formation.
Interference with metabolic pathways involved in IGMTs enhanced the callus formation of plants
The mechanism by which IGMT acts as an enzyme is likely to be related to its substrates or products. Unfortunately, none of the products catalyzed by IGMT, including 4MOI3M and 1MOI3M, could be obtained by commercial means. In IGS metabolic pathway, the cytochrome P450 CYP83B1, which catalyzes the first committed step in IGS biosynthesis, could be competitively inhibited by Tryptamine treatment (Bak 2001; Celenza 2001). To analyze the mechanism of action of IGMT affecting callus initiation, we interfered with the IGS metabolic pathway by Tryptamine treatment, which was expected to reduce the contents of indole glucosinolates in plants (Bak 2001). WT and igmts grown on 1/2 MS medium did not show significant differences after 5 days of treatment with different concentrations of Tryptamine (Fig. 4A). In contrast, the callus formation ability of plants was significantly enhanced after 5 days of Tryptamine treatment in CIM medium. The treatment with 100 and 200 µM of Tryptamine resulted in a 22.6% and 20.9% increase in the number of callus initiation per unit root length, respectively (Fig. 4B, C), compared to the DMSO control. This result seems to indicate that blocking IG synthesis facilitates the formation of healing wounds, but tryptamine is also one of the substrates for IAA synthesis and tryptamine treatment will lead to an increase in IAA content. Therefore, the relationship between IG synthesis and healing wound formation needs to be further investigated.
Fig. 4.
Interference with the synthesis of indole glucosinolate enhances plant callus formation. A The callus-forming phenotype of 7-day-old WT and igmts seedlings cultured on 1/2 MS without or with 100 and 200 µM Tryptamine treatment for 5 days. B The callus-forming phenotype of 7-day-old WT seedlings cultured on CIM without or with 100 and 200 µM Tryptamine treatment for 5 days. C Quantification of the callus initiation on the primary roots of seedlings cultured on CIM without or with 100 and 200 µM Tryptamine for 5 days. Scale bar = 10 mm. Error bars = ± SD, n > 12
By analyzing transcriptome data from our previous studies (Xu et al. 2017), we found that the transcription factor MYB122, which positively regulates indole glucosinolate synthesis genes and indole glucosinolates content in vivo (Frerigmann and Gigolashvili 2014; Yu et al. 2021), was induced by LBD29. We then generated p35S:MYB122 transgenic plants and found that they exhibited different degrees of spontaneous callus initiation phenotypes on MS medium in the absence of exogenous hormones (Fig. 5). Consistent with the description of IGMT overexpression plants above, the callus formation phenotypes of transgenic plants were similarly classified as strong, medium, and weak types. Of these, strong, medium, and weak type phenotypes accounted for 1.7%, 10.9%, and 87.4%, respectively. This result suggests that enhancement of the IGS metabolic pathway may promote callus formation.
Fig. 5.
Ectopic expression of MYB122 triggers callus formation
The phenotype of varying degrees of the autonomous callus formation in the T1 transgenic seedlings overexpressing MYB122. The callus formation phenotypes were grouped into three categories: strong (S), medium (M) and weak (W) type. After transfer to B5 medium, strong type plants showed a phenotype of swollen and thickened roots and abnormally curled leaves within 10 days, and formed visible callus tissues in root, stem and leaf tissues within 20 days. The medium type plants showed swollen and thickened roots and abnormal leaf development, but no visible callus tissue within 20 days. The weak type plants showed no developmental abnormalities within 10 days, but developed a lateral root increase phenotype after 20 days. And the statistics on the number of plants with different types of callus formation phenotypes. Scale bars = 5 mm (seedlings)/100 µm (enlarged images).
IGMT—mediated alterations in callus formation were accompanied by alterations in ROS homeostasis
The substrate indole glucosinolate (IGS) of IGMTs has been reported to attenuate FB1 (fumonisin B1)-induced reactive oxygen species (ROS) accumulation and subsequent programmed cell death (Zhao et al. 2015). The de novo shoot initiation was regulated by thioredoxin-dependent redox modification of ROS homeostasis (Zhang et al. 2017). The ROS balance between H2O2 and ·O2− is essential for stem cell maintenance and differentiation, in which high ·O2− accumulation is important to stem cells, whereas H2O2 is distributed in the differentiating cells (Zeng et al. 2017).
To analyze the mechanism by which IGMTs affect callus formation, we analyzed the reactive oxygen species status in IGMT mutants and overexpression plants. We first examined the distribution and accumulation of ·O2− in WT, igmt mutant and weak Pro35S:IGMT3 transgenic lines treated on CIM medium for 0, 4, and 7 days by nitroblue tetrazolium (NBT) staining which is specific for ·O2− staining. As shown in Fig. 6A, NBT staining mainly accumulated in the stele of the root elongation zone, whereas the distribution was relatively weak in the root mature zone. In addition, ·O2− accumulation was also observed in the apical meristem and leaves in Arabidopsis. After CIM treatment for 4 days, a weak signal of NBT was detected in the formed callus. Compared with WT, the content of ·O2− was higher in Pro35S:IGMT3 transgenic plant, while the signal was decreased in igmt mutants. At the same time, we examined the expression of genes involved in ROS biosynthesis and metabolism among WT, igmt mutant, and Pro35S:IGMT3 transgenic plant. The results showed that Alternative Oxidase (AOX) genes AOX1A, AOX1C, AOX1D, and AOX2, which function in ·O2− biosynthesis process, were upregulated in Pro35S:IGMT3 transgenic plant, however, in mutant lines, these genes were repressed compared with WT (Fig. 6B). On the other hand, Peroxidase (PER) genes PER52, PER58, PER15, and PER49, which fuction in decomposing the H2O2 to water and resulting in low-level H2O2 and high ·O2− accumulation (Zeng et al. 2017), were upregulated in Pro35S:IGMT3 transgenic plant, and downregulated in igmt mutants (Fig. 6B). These results implied that IGMT3 could promote ·O2− synthesis and accumulation by inducing the expression of alternative oxidase and peroxidase coding genes.
Fig. 6.
IGMT3 promoted ·O2− accumulation. A NBT staining of WT, igmts, and Pro35S:IGMT3 transgenic seedlings. 7-day-old WT, igmts, and Pro35S:IGMT3 transgenic seedlings incubated on CIM for 0 d, 4 d, and 7 d were staining for 1 h. Insets show enlarged images of the root meristematic zone and mature zone incubated on CIM for 0 d and 4 d, the right images show the plants treated on CIM for 7 d. Scale bars = 5 mm (seedlings)/100 µm (enlarged images). B Relative expression of genes involved in O2·− biosynthesis and metabolism in 7-day-old WT, igmts, and Pro35S:IGMT3 transgenic seedlings. Error bars = ± SD, n = 3 biological replicates
To further investigate whether the ·O2− accumulation affected the callus formation, (N, N′ - dimethylthiourea) DMTU, which is a scavenger of ·O2−, was applied to the callus induction medium. After 7 days of DMTU treatment, we have observed that the ability of the callus formation of WT, igmts, and Pro35S:IGMT3 transgenic seedlings were weakened significantly (Fig. 7A and B). Further, we analyze the peroxidase repressor mutant upb1-1, which accumulated a high level of ·O2− by reducing the H2O2 level through peroxidase activity (Zeng et al. 2017). As expected, the upb1-1 mutant exhibited enhanced capacity in callus formation compared to WT, conversely, the callus formation was attenuated in Pro35S:UPB1 transgenic plant (Fig. 7C, D), indicating that ·O2− is crucial for callus formation. These results suggested that overexpression of IGMT3 mediated callus formation were accompanied by alterations in ROS homeostasis.
Fig. 7.
·O2− enhanced the callus formation ability. A 5-day-old WT, igmts, Pro35S:IGMT3 seedlings cultured on CIM with DMSO or CIM with 10 mM DMTU for 7 days. B Quantification of the callus initiation on the primary roots of seedlings cultured on CIM mock medium or CIM with DMTU for 7 days. Scale bar = 10 mm. Error bars = ± SD, n > 12. C Callus-forming phenotype of 5-day-old WT, Pro35S:UPB1 and upb1 seedlings cultured on CIM for 5 days. D Quantification of the callus initiation on the primary roots of seedlings cultured on CIM for 4 days. Scale bar = 10 mm. Error bars = ± SD, n > 12
Discussion
Four LBD genes (LBD16, LBD17, LBD18, and LBD29) integrate multiple upstream signals in plant callus formation and are regulated by multiple upstream factors. Under in vitro culture conditions, exogenous auxin signals activate the expression of four LBD genes through Auxin Response Factor7 (ARF7) and ARF19 (Fan et al. 2012; Lee et al. 2009; Okushima et al. 2007). Wuschel Related Homeobox11 WOX11 (WOX11) and WOX12 are involved in the first-step cell fate transformation for de novo root organogenesis by activating LBD16 and LBD29 expression (Liu et al. 2014). In addition, two MYB transcription factors, MYB94 and MYB96, additively inhibit callus formation via directly repressing LBD29 expression (Dai et al. 2019). As transcription factors, previous studies have drafted the downstream molecular events regulated by LBD29 and identified a large number of potential target genes (Xu et al. 2017). Except for LAX3, a downstream gene of LBD29 (Porco et al. 2016), and EXP14 and E2Fa, downstream genes of LBD18 (Berckmans et al. 2011; Lee et al. 2012), the role of most LBDs’ target genes in callus formation has not been fully analyzed previously.
Based on our previous study (Xu et al. 2017), IGMT2 and IGMT3 are target genes of LBD29. GFP fluorescence observation and GUS staining showed that the nucleus and cell membrane-localized IGMT genes were mainly expressed in the maturation and elongation zones of the root, while less expressed in the meristematic zone. By the time-course expression analysis of IGMT genes after callus induction using qPCR and GUS staining, we found that the four genes were mainly induced at the early stage of callus initiation and recovered rapidly. Moreover, IGMT3 was most strongly induced among the four genes. These results suggest that the rapid response of the IGMT gene family to LBDs, with IGMT3 as the major player, may be an important early event in callus induction.
Although IGMT2 and IGMT3 overexpressing plants exhibit varying degrees of autonomous callus initiation phenotypes, there is no previous definitive genetic evidence before. Due to the redundancy of the IGMT1-4 genes, none of the single mutants exhibited a significant defect in callus formation. And the tight arrangement of the four genes on the chromosome makes it almost impossible to obtain multiple mutants by hybridization. In this study, we obtained igmt quadruple mutant using CRISPR/Cas9 technology based on the common sequence of four genes, which confirmed the role of IGMTs in callus formation.
As the enzyme catalyzes IGS biosynthesis, it is logical that IGMTs act through its substrates IGS. Although these products (4MOI3M and 1MOI3M) were not available by commercial means, we demonstrated to some extent that the substrates and metabolic pathway, that IGMTs involved, have an important role in callus formation by overexpressing MYB122, a regulatory gene of this pathway. Of course, further efforts are needed to clarify the specific substances that work.
Extensive work in recent years has revealed the important role of ROS homeostasis in maintaining cell fate and regulating plant regeneration (Tsukagoshi et al. 2010; Zeng et al. 2017; Zhang et al. 2017). Genes encoding class III PERs, which are mainly involved in the production of plastid exosomes ROS and callose formation during plant response to stress (Spruyt et al. 2016), are induced by LBD29 (Xu et al. 2017). Coincidentally, the IGS themselves have immunological effects closely related to stress-induced ROS synthesis (Chhajed et al. 2020; Iwase et al. 2021; Ji et al. 2020; Tao et al. 2022; Yi et al. 2022). The results of NBT staining showed that the content of ·O2− in CIM-treated plants was positively correlated with the expression of IGMTs to some extent. Consistent with this, genes encoding ·O2− biosynthesis enzymes (AOX1A, AOX1C, AOX1D, and AOX2) as well as PER family genes (PER52, PER58, PER15, and PER49) were up-regulated in IGMT overexpressing plants and down-regulated in igmt mutants. As reported by Zeng et al. (2017) an increasing level of ·O2− signifies the stem cell state of plant cells, while a high concentration of H2O2, which negatively regulates ·O2− synthesis, represents the termination of stem cell fate. In addition, the reduction of ·O2− content by DMTU treatment or enhancing the function of peroxidase repressor UBP1, negatively regulates the callus formation of all plants, including WT, igmts, Pro35S:IGMT3 seedlings. Therefore, IGMTs-regulated plant callus formation may be related to ROS homeostasis.
Hormone-induced callus formation and wounding-induced callus formation are currently the most studied mechanisms of plant callus formation. However, there is very limited understanding of the intrinsic linkage between the two pathways. The expression of IGMT1-4 is induced by multiple wound-like factors (Guan and Nothnagel 2004; Nagata et al. 2005; Tsukagoshi et al. 2010; Xu et al. 2016). And these genes in turn can spontaneously induce callus formation as target genes of LBD29. These results imply that the responding of IGMT1-4 genes can serve as an important node for hormonal and wound-induced callus formation.
Conclusions
In conclusion, we here provide valid evidence for the involvement of IGS metabolic pathway genes IGMT1-4 as target genes of LBD29 in callus formation. Enhancement of IGS metabolic pathway seems to promote callus formation. And IGMT1-4-mediated callus formation affects ROS homeostasis in vivo.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Author contributions
Conceptualization, HC and XZ; investigation, HC, FL, ZH and YZ; writing—original draft preparation, HC; writing—review and editing, XZ and XG; project administration, XZ; funding acquisition, HC and XZ All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Natural Science Foundation of Shanxi Province (20210302123343), The Program for Scientific and Technological Innovation of Higher Education Institutions in Shanxi (2021L378), Industry-University-Research Project of Shanxi Datong University (2020CXZ16, 2021CXZ6), Shanxi Datong University research project - Yungang study (2021YGZX45), Shanxi Datong University Students Innovation and Entrepreneurship Project (XDC2022147, XDC2022157).
Data availability
The data sets generated and analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Conflict of interest
The authors declare no conflict of interest.
Consent to participate
All authors participate to finish the work.
Consent for publication
All authors agreed for publication.
Ethics approval
Compliance with ethical standards.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Bak S. CYP83B1, a cytochrome P450 at the metabolic branch point in auxin and indole glucosinolate biosynthesis in arabidopsis. Plant Cell. 2001;13:101–111. doi: 10.1105/tpc.13.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bednarek P, Pislewska-Bednarek M, Svatos A, Schneider B, Doubsky J, Mansurova M, Humphry M, Consonni C, Panstruga R, Sanchez-Vallet A, Molina A, Schulze-Lefert P. A glucosinolate metabolism pathway in living plant cells mediates broad-spectrum antifungal defense. Science. 2009;323(5910):101–106. doi: 10.1126/science.1163732. [DOI] [PubMed] [Google Scholar]
- Berckmans B, Vassileva V, Schmid S, Maes S, Parizot B, Naramoto S, Magyar Z, Kamei C, Koncz C, Bögre L, Persiau G, Jaeger GD, Friml J, Simon R, Beeckman T, Veylder LD. Auxin-dependent cell cycle reactivation through transcriptional regulation of Arabidopsis E2Fa by lateral organ boundary proteins. Plant Cell. 2011;23(10):3671–3683. doi: 10.1105/tpc.111.088377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro B, Citterico M, Kimura S, Stevens DM, Wrzaczek M, Coaker G. Stress-induced reactive oxygen species compartmentalization, perception and signalling. Nat Plants. 2021;7:403–412. doi: 10.1038/s41477-021-00887-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celenza JL. Metabolism of tyrosine and tryptophan-new genes for old pathways. Curr Opin Plant Biol. 2001;4(3):234–240. doi: 10.1016/S1369-5266(00)00166-7. [DOI] [PubMed] [Google Scholar]
- Chhajed S, Mostafa I, He Y, Abou-Hashem M, El-Domiaty M, Chen S. Glucosinolate biosynthesis and the glucosinolate–myrosinase system in plant defense. Agronomy. 2020;10(11):1786. doi: 10.3390/agronomy10111786. [DOI] [Google Scholar]
- Clay N, Adio A, Denoux C, Jander G, Ausubel F. Glucosinolate metabolites required for an Arabidopsis innate immune response. Science. 2009;323(5910):95–101. doi: 10.1126/science.1164627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough SJ, Bent A. Floral dip: a simplified method for agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998;16(6):735–743. doi: 10.1046/j.1365-313x.1998.00343.x. [DOI] [PubMed] [Google Scholar]
- de Vos M, Kriksunov KL, Jander G. Indole-3-acetonitrile production from indole glucosinolates deters oviposition by Pieris rapae. Plant Physiol. 2008;146(3):916–926. doi: 10.1104/pp.107.112185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai XH, Liu N, Xiang FN, Liu ZH. MYB94 and MYB96 additively inhibit callus formation via directly repressing LBD29 expression in Arabidopsis thaliana. Plant Sci. 2019;293:110323. doi: 10.1016/j.plantsci.2019.110323. [DOI] [PubMed] [Google Scholar]
- Fan J, Crooks C, Creissen G, Hill L, Fairhurst S, Doerner P, Lamb C. Pseudomonas sax genes overcome aliphatic isothiocyanate-mediated non-host resistance in Arabidopsis. Science. 2011;331(6021):1185–1188. doi: 10.1126/science.1199707. [DOI] [PubMed] [Google Scholar]
- Fan M, Xu C, Xu K, Hu Y. Lateral organ boundaries domain transcription factors direct callus formation in Arabidopsis regeneration. Cell Res. 2012;22(7):1169–1180. doi: 10.1038/cr.2012.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of aging. Nature. 2000;408(6809):239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- Frerigmann H, Gigolashvili T. MYB34, MYB51, and MYB122 distinctly regulate indolic glucosinolate biosynthesis in Arabidopsis thaliana. Mol Plant. 2014;7(5):814–828. doi: 10.1093/mp/ssu004. [DOI] [PubMed] [Google Scholar]
- Guan Y, Nothnagel EA. Binding of arabinogalactan proteins by Yariv phenylglycoside triggers wound-like responses in Arabidopsis cell cultures. Plant Physiol. 2004;135(3):1346–1366. doi: 10.1104/pp.104.039370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halkier BA, Gershenzon J. Biology and biochemistry of glucosinolates. Annu Rev Plant Biol. 2006;57:303–333. doi: 10.1146/annurev.arplant.57.032905.105228. [DOI] [PubMed] [Google Scholar]
- Ikeuchi M, Sugimoto K, Iwase A. Plant callus: mechanisms of induction and repression. Plant Cell. 2013;25(9):3159–3173. doi: 10.1105/tpc.113.116053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwase A, Kondo Y, Laohavisit A, Takebayashi A, Ikeuchi M, Matsuoka K, Asahina M, Mitsuda N, Shirasu K, Fukuda H, Sugimoto K. WIND transcription factors orchestrate wound-induced callus formation, vascular reconnection and defense response in Arabidopsis. New Phytol. 2021;232:734–752. doi: 10.1111/nph.17594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang YL, Sharkis SJ. A low level of reactive oxygen species selects for primitive hematopoietic stem cells that may reside in the low-oxygenic niche. Blood. 2007;110(8):3056–3063. doi: 10.1182/blood-2007-05-087759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji R, Lei JX, Chen IW, Sang W, Zhu-Salzman K. Cytochrome P450s CYP380C6 and CYP380C9 in green peach aphid facilitate its adaptation to indole glucosinolate-ediated plant defense. Pest Manag Sci. 2020;77:148–158. doi: 10.1002/ps.6002. [DOI] [PubMed] [Google Scholar]
- Kim JH, Jander G. Myzus persicae (green peach aphid) feeding on arabidopsis induces the formation of a deterrent indole glucosinolate. Plant J. 2007;49(6):1008–1019. doi: 10.1111/j.1365-313X.2006.03019.x. [DOI] [PubMed] [Google Scholar]
- Lee HW, Kim M-J, Kim NY, Lee SH, Kim J. LBD18 acts as a transcriptional activator that directly binds to the EXPANSIN14 promoter in promoting lateral root emergence of Arabidopsis. Plant J. 2012;73:212–224. doi: 10.1111/tpj.12013. [DOI] [PubMed] [Google Scholar]
- Lee HW, Kim NY, Lee DJ, Kim J. LBD18/ASL20 regulates lateral root formation in combination with LBD16/ASL18 downstream of ARF7 and ARF19 in Arabidopsis. Plant Physiol. 2009;151:1377–1389. doi: 10.1104/pp.109.143685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Sheng L, Xu Y, Li J, Yang Z, Huang H, Xu L. WOX11 and 12 are involved in the first-step cell fate transition during de novo root organogenesis in Arabidopsis. Plant Cell. 2014;26:1081–1093. doi: 10.1105/tpc.114.122887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludin A, Gur-Cohen S, Golan K, Kaufmann K, Itkin T, Medaglia C, Lu XJ, Ledergor G, Kollet O, Lapidot T. Reactive oxygen species regulate hematopoietic stem cell self-renewal, migration and development, as well as their bonemarrow microenvironment. Antioxid Redox Sign. 2014;21:1605–1619. doi: 10.1089/ars.2014.5941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mignolet-Spruyt L, Xu EJ, Idänheimo N, Hoeberichts FA, Mühlenbock P, Brosche M, Breusegem FV, Kangasjärvi J. Spreading the news: subcellular and organellar reactive oxygen species production and signalling. J Exp Bot. 2016;67(13):3831–3844. doi: 10.1093/jxb/erw080. [DOI] [PubMed] [Google Scholar]
- Mittler R, Zandalinas SI, Fichman Y, Van Breusegem F. Reactive oxygen species signalling in plant stress responses. Nat Rev Mol Cell Biol. 2022;23(10):663–679. doi: 10.1038/s41580-022-00499-2. [DOI] [PubMed] [Google Scholar]
- Nagata T, Yamada H, Du Z, Todoriki S, Kikuchi S. Microarray analysis of genes that respond to γ-irradiation in Arabidopsis. J Agric Food Chem. 2005;53(4):1022–1030. doi: 10.1021/jf0486895. [DOI] [PubMed] [Google Scholar]
- Okushima Y, Fukaki H, Onoda M, Theologis A, Tasaka M. ARF7 and ARF19 regulate lateral root formation via direct activation of LBD/ASL genes in Arabidopsis. Plant Cell. 2007;19:118–130. doi: 10.1105/tpc.106.047761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedras MSC, Yaya E, Glawischnig E. ChemInform abstract: the phytoalexins from cultivated and wild crucifers: chemistry and biology. Nat Prod Rep. 2011;28:1381–1405. doi: 10.1039/c1np00020a. [DOI] [PubMed] [Google Scholar]
- Pfalz M, Mikkelsen MD, Bednarek P, Olsen CE, Halkier BA, Kroymann J. Metabolic engineering in Nicotiana benthamiana reveals key enzyme functions in Arabidopsis indole glucosinolate modification. Plant Cell. 2011;23(2):716–729. doi: 10.1105/tpc.110.081711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfalz M, Mukhaimar M, Perreau F, Kirk J, Hansen CIC, Olsen CE, Agerbirk N, Kroymann J. Methyl transfer reactions in glucosinolate biosynthesis mediated by indole glucosinolate O-methyltransferase 5. Plant Physiol. 2016;172(4):2190–2203. doi: 10.1104/pp.16.01402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfalz M, Vogel H, Kroymann J. The gene controlling the Indole Glucosinolate Modifier1 quantitative trait locus alters indole glucosinolate structures and aphid resistance in Arabidopsis. Plant Cell. 2009;21(3):985–999. doi: 10.1105/tpc.108.063115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porco S, Larrieu A, Du YJ, Gaudinier A, Goh T, Swarup K, Swarup R, Kuempers B, Bishopp A, Lavenus J, Casimiro I, Hill K, Benkova E, Fukaki H, Brady SM, Scheres B, Péret B, Bennett MJ. Lateral root emergence in Arabidopsis is dependent on transcription factor LBD29 regulating auxin influx carrier LAX3. Development. 2016;143(18):dev.136283. doi: 10.1242/dev.136283. [DOI] [PubMed] [Google Scholar]
- Reinert J, Backs D. Control of totipotency in plant cells growing in vitro. Nature. 1969;220(5174):1340–1341. doi: 10.1038/2201340a0. [DOI] [PubMed] [Google Scholar]
- Rogers L, Dubos C, Cullis I, Surman C, Poole M, Willment J, Mansfield S, Campbell M. Light, the circadian clock, and sugar perception in the control of lignin biosynthesis. J Exp Bot. 2005;56(416):1651–1663. doi: 10.1093/jxb/eri162. [DOI] [PubMed] [Google Scholar]
- Shang BS, Xu CY, Zhang XX, Cao HF, Xin W, Hu YX. Very-long-chain fatty acids restrict regeneration capacity by confining pericycle competence for callus formation in Arabidopsis. P Natl Acad Sci USA. 2016;113(18):5101–5106. doi: 10.1073/pnas.1522466113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skoog F, Miller CO. Chemical regulation of growth and organ formation in plant tissues cultured in vitro. Symp Soc Exp Biol. 1957;11:118–130. [PubMed] [Google Scholar]
- Sugimoto K, Gordon SP, Meyerowitz EM. Regeneration in plants and animals: dedifferentiation, transdifferentiation, or just differentiation? Trends Cell Biol. 2011;21(4):212–218. doi: 10.1016/j.tcb.2010.12.004. [DOI] [PubMed] [Google Scholar]
- Sugimoto K, JiaoY L, Meyerowitz EM. Arabidopsis regeneration from multiple tissues occurs via a root development pathway. Dev Cell. 2010;18(3):463–471. doi: 10.1016/j.devcel.2010.02.004. [DOI] [PubMed] [Google Scholar]
- Sugiyama M. Partnership for callusing. Nat Plants. 2018;4(2):69–70. doi: 10.1038/s41477-018-0104-2. [DOI] [PubMed] [Google Scholar]
- Sun JY, Sonderby IE, Halkier BA, Jander G, de Vos M. Non-volatile intact indole glucosinolates are host recognition cues for ovipositing Plutella xylostella. J Chem Ecol. 2009;35(12):1427–1436. doi: 10.1007/s10886-009-9723-4. [DOI] [PubMed] [Google Scholar]
- Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol. 2013;30(12):2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao H, Miao HY, Chen LL, Wang MY, Xia CC, Zeng W, Sun B, Zhang F, Zhang SQ, Li CY. WRKY33-mediated indolic glucosinolate metabolic pathway confers resistance against Alternaria Brassicicola in Arabidopsis and Brassica crops. J Integr Plant Biol. 2022;64:1007–1019. doi: 10.1111/jipb.13245. [DOI] [PubMed] [Google Scholar]
- Tsukagoshi H, Busch W, Benfey PN. Transcriptional regulation of ROS controls transition from proliferation to differentiation in the root. Cell. 2010;143(4):606–616. doi: 10.1016/j.cell.2010.10.020. [DOI] [PubMed] [Google Scholar]
- Xu CY, Cao HF, Xu EJ, Zhang SQ, Hu YX. Genome-wide identification of Arabidopsis LBD29 target genes reveals the molecular events behind auxin-induced cell reprogramming during callus formation. Plant Cell Physiol. 2017;59:749–760. doi: 10.1093/pcp/pcx168. [DOI] [PubMed] [Google Scholar]
- Xu CY, Cao HF, Zhang QQ, Wang HZ, Xin W, Xu EJ, Zhang SQ, Yu RX, Yu DX, Hu YX. Control of auxin-induced callus formation by bZIP59–LBD complex in Arabidopsis regeneration. Nat Plants. 2018;4:108–115. doi: 10.1038/s41477-017-0095-4. [DOI] [PubMed] [Google Scholar]
- Xu CY, Hu YX. The molecular regulation of cell pluripotency in plants. Abiotech. 2020 doi: 10.1007/s42994-020-00028-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Meng J, Meng XZ, Zhao YT, Liu JM, Sun TF, Liu YD, Wang QM, Zhang SQ. Pathogen-responsive MPK3 and MPK6 reprogram the biosynthesis of indole glucosinolates and their derivatives in Arabidopsis immunity. Plant Cell. 2016;28:1144–1162. doi: 10.1105/tpc.15.00871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi SY, Lee M, Park SK, Lu L, Lee G, Kim S-G, Kang S-Y, Lim YP. Jasmonate regulates plant resistance to Pectobacterium brasiliense by inducing indole glucosinolate biosynthesis. Front Plant Sci. 2022;13:964092. doi: 10.3389/fpls.2022.964092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y, Xu MM, Ding XH, Chu ZH, Liu HF. Activating the MYB51 and MYB122 to upregulate the transcription of glucosinolates biosynthesis genes by copper ions in Arabidopsis. Plant Physiol Bioch. 2021;162:496–505. doi: 10.1016/j.plaphy.2021.03.025. [DOI] [PubMed] [Google Scholar]
- Zeng J, Dong ZC, Wu HJ, Tian ZX, Zhao Z. Redox regulation of plant stem cell fate. EMBO J. 2017;36(19):e201695955. doi: 10.15252/embj.201695955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Zhang TT, Liu H, Shi DY, Wang M, Bie XM, Li XG, Zhang XS. Thioredoxin- mediated ros homeostasis explains natural variation in plant regeneration. Plant Physiol. 2017;176(3):2231. doi: 10.1104/pp.17.00633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao YT, Wang JS, Liu YY, Miao HY, Cai CX, Shao ZY, Guo RF, Sun B, Jia CG, Zhang LP, Gigolashvili T, Wang QM. Classic myrosinase-dependent degradation of indole glucosinolate attenuate fumonisin B1-induced programmed cell death in Arabidopsis. Plant J. 2015;81:920–933. doi: 10.1111/tpj.12778. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data sets generated and analyzed during the current study are available from the corresponding author on reasonable request.