Abstract
Background
Effect of antibacterials on mucociliary system and clinical outcome of chickens with mixed viral respiratory conditions is not properly addressed.
Objective
We evaluated enrofloxacin effects on clinical parameters and mucociliary system of broilers challenged with H9N2/IB viruses.
Methods
Broilers (105), at the age of 25 days, were randomly allocated into three groups: Group 1 (negative control), no treatment; Group 2 (positive control [PC]) challenged by intranasal and intraocular route. Group 3 (antibiotic [AB]‐treated) challenged and also received enrofloxacin started after manifestation of clinical signs (day 2 post‐challenge [pc]) and continued for 5 days.
Results
Administration of AB was not associated with appreciable changes in body weight, feed conversion ratio (FCR) or the severity of clinical signs although it slightly reduced mortality rate as compared to PC group (p > 0.05). Virus shedding period and number of virus positive tracheal and caecal tonsil samples were also statistically similar between PC and AB groups. In necropsy, the most profound effect of AB was decreased pleuropneumonia severity score on day 12 pc. Histopathological lesion scores were statistically the same between PC and AB groups. However, the administration of AB increased the number of tracheal goblet cells, with no effect on ciliostasis.
Conclusions
We found a weak positive effect of enrofloxacin administration in H9N2/IB‐infected chickens. Considering the risks of AB treatment in broiler chickens, the results of this small‐scale study do not encourage the benefit of enrofloxacin use in these viral diseases.
Keywords: antibacterial, avian influenza, broiler, infectious bronchitis, mucociliary system
We found a weak positive effect of enrofloxacin administration in H9N2/IB‐infected chickens. Considering the risks of antibiotic treatment in broiler chickens, the results of this small‐scale study do not encourage the benefit of enrofloxacin use in these viral diseases, although performing future risk–benefit balance studies on a large number of birds and for longer periods following infection is suggested.
1. INTRODUCTION
Low pathogenic avian influenza (LPAI) H9N2 virus and infectious bronchitis virus (IBV) are both considered important culprits for respiratory problems even in vaccinated broilers. Co‐infection with these viruses is often observed, which results in the exacerbation of respiratory condition with striking economic loss (Belkasmi et al., 2020). Secondary bacterial infections following damage of the respiratory tract are common and put more burdens on the bird body system, which leads to an increased rate of mortality among infected birds (Hassan et al., 2017).
Regarding the urgent need for the prudent use of antibacterials, the administration of these agents in viral poultry diseases is not supported by organizations like FAO (2021Magnusson). However, broad‐spectrum agents may be prescribed for flocks with IB or LPAI to reduce the sequela of secondary bacterial infections and subsequent precipitation of clinical signs (Ennaji et al., 2020; Swayne et al., 2021). Immunosuppression occurs in bird flocks infected with H9N2 LPAI (Yehia et al., 2023); consequently, opportunistic bacteria like Escherichia coli can aggravate the clinical condition, and therefore, antibacterial therapy may help in controlling the disease and reducing economic loss (Bano et al., 2003; Zhang et al., 2020). On the other hand, extensive use of antibacterials increases the risk of antimicrobial resistance as well as drug residue violations in edible tissues of the chicken. Acquiring knowledge on the risk–benefit balance of antibacterial use in order to prevent secondary bacterial infections in poultry flocks with viral diseases is highly demanded.
Apart from direct effects on bacteria, antibacterials may show ancillary effects that are important determinants of their therapeutic outcome. For instance, immunomodulatory effects of tilmicosin, florfenicol and enrofloxacin are well‐defined. Khalifeh et al. (2009) showed that these agents reduce the humoural immune response while accelerating cell‐mediated immune response in chickens vaccinated against Newcastle disease.
Innate immunity is also important in confronting respiratory pathogens and the respiratory system exploits cilia, mucus and phagocytic cells as parts of the innate immune system to fight back against infections (Gray et al., 2021); in fact, proper functions of cilia in the upper respiratory tract and mucociliary clearance are important as the first line of defence against opportunistic bacteria (Jackwood et al., 2015).
It has been previously described that certain macrolide antibiotics (ABs) (Takeyama et al., 1993) as well as ciprofloxacin (Takemura et al., 1996) increase the ciliary beat frequency of rabbit‐cultured tracheal epithelium, whereas macrolides can decrease mucus secretion by the nasal epithelium of rats (Shimizu et al., 2003). In chickens with IB, epithelial hyperplasia, ciliary loss and necrosis, and inflammatory cell infiltration, including heterophils, macrophages and natural killer cells, are observed (Zhang et al., 2021). Discontinuation of respiratory epithelium and loss of cilia are reported in chickens challenged with H9N2 LPAI virus (Jaleel et al., 2017).
There is no previous experiment on the effect of antibacterials on parameters related to mucociliary system and outcome of disease in chickens with mixed viral respiratory conditions. In the current study, we evaluated the effects of enrofloxacin administration to broilers challenged with H9N2/IB viruses on birds’ performance parameters, clinical signs, gross and histopathological lesions, virus shedding as well as number of tracheal goblet cells and ciliary activity scores.
2. MATERIALS AND METHODS
2.1. Study design
One hundred and five Hubbard broiler chickens from both sexes were purchased at the age of one day. Birds were randomly allocated into three equal groups (n = 35 each in five replicates) and transferred into separate rooms under biosecurity conditions. Birds were reared in cages as indicated by the Hubbard rearing manual and had free access to feed and water during the experiment. No vaccination was performed during the experiment. At the age of 25 days, birds were weighed and treated as follows: Group 1 (negative control [NC] birds) received no treatment; Group 2 (positive control [PC] birds) challenged with 100 µL of allantoic fluid containing 105EID50/mL of IB virus (793/B serotype IRFIBV32) as well as 100 µL of allantoic fluid containing 107.1EID50/mL of H9N2 LPAI (A/Chicken/Iran/772/1998). For both viruses, 50 µL of the fluid was administered by intranasal and 50 µL by intraocular route. Group 3 (AB‐treated birds) was challenged as above and also received enrofloxacin (Kimiaenro+Na® 10%, Kimia Faam Pharmaceutical Co.) at the dosage of 1 mL/L of drinking water as indicated by the manufacturer. Enrofloxacin administration was started after the manifestation of clinical signs (day 2 post‐challenge [pc]) and continued for 5 consecutive days.
Procedures used in the present study are approved by institutional ethical committee (95GCU2M163723) and are in accordance with Directive 2010/63/EU.
2.2. Performance parameters and clinical signs
On days 1, 3, 6, 9 and 12 pc, the severity of the signs was scored in a scale of 1–4. Normal birds with no sign scored 1, score 2 was assigned to birds with watery eyes or mucus in their nares, the presence of watery eyes and mucus in nares scored 3 and finally birds with watery eyes and mucus in nares as well as tracheal rales scored 4 (Jackwood et al., 2010). Birds were weighed and feed consumption and feed conversion ratio (FCR) were determined for each group at the end of the experiment (day 12 pc or at the age of 37 days). Mortality was also recorded daily.
2.3. Gross and histopathology
On days 3, 6, 9 and 12 pc, five birds from each group were randomly selected and euthanized. Carcasses of these birds as well as birds that died during the experiment were grossly evaluated for lesions. Lesions in the respiratory system of euthanized birds were scored from 1 to 4 (1 = no lesion, 2 = mild lesions, 3 = moderate lesion and 4 severe lesions were observed). Lesions for scoring were included of tracheal hyperaemia, tracheal exudates, tracheal casts, casts in syrinx, lung congestion, pleuropneumonia and air sacculitis (Jaleel et al., 2017 with little modification). Changes in liver, kidney and spleen were considered qualitatively.
Middle part of the trachea was dissected, and after formalin fixation, haematoxylin and eosin‐stained slides were prepared and examined under light microscope for histopathological changes. The severity of lesions was scored based on the severity of epithelial changes, lymphocytes infiltration, loss of cilia and oedema in lamina propria from 1 to 4 (1 = least severe and 4 most severe lesions) (Amanollahi et al., 2021).
2.4. Goblet cell counting
To count the number of goblet cells, three slides of tracheal tympanum from each bird were prepared on days 3 and 6 pc and stained with periodic acid–Schiff method. The numbers of goblet cells were counted in 5 fields of each slide at the magnification of 40, and the mean of 15 counts was considered the number of goblet cell/field for each sample (Abbasnia et al., 2020).
2.5. Ciliostasis test
After washing trachea in Hank's buffer, tracheal rings with the thickness of 2 mm were prepared from upper (three rings), middle (four rings) and lower (three rings) parts of the trachea on days 3 and 6 pc. The rings were placed in micro titre plates containing Dulbecco's Modified Eagle Medium and were shaken gently at 37°C for few minutes. The activity of cilia was examined under an inverted microscope and scored as 0 = 100%, 1 = 75%, 2 = 50%, 3 = 25% and 4 = 0% ciliary activity (Snyder et al., 1983).
2.6. Virus detection period
On days 3, 6, 9 and 12 pc, samples from the middle part of trachea and caecal tonsils of five birds from each group were collected under sterile conditions and kept at −70°C. Fifty milligrams of each sample were homogenized in PBS and RNA extraction was performed by using RNX‐PLUS (Sinaclon). Extracted RNA was quantified by a nanodrop spectrophotometer (Eppendorf BioSpectrometer, Eppendorf International). PrimeScript RT Reagent kit (Takara Bio.) was used to build cDNA. The primer pair used for each virus is shown in Table 1. The duplex RT PCR was performed by using Red Load Taq Master Mix (Jena Bioscience). The reaction mixture contained master mix: 12.5 µL, Taq polymerase: 0.5 µL, Primer H9F (10 pmol/µL): 1 µL, Primer H9R (10 pmol/µL): 1 µL, Primer SX3F (10 pmol/µL): 1 µL, Primer SX4R (10 pmol/µL): 1 µL, DW: 4 µL, cDNA: 4 µL in a total volume of 25 µL. The PCR condition was as follows: primary denaturation 3 min at 94°C, denaturation 30 s at 94°C, binding 30 s at 50°C, amplification 35 s at 72°C and final amplification 10 min at 72°C. Allantoic fluids that contained H9N2 or IB viruses were used as PCs. Four microlitres of the product were used for 2% agarose gel electrophoresis.
TABLE 1.
Primer sequences used for building cDNA of infectious bronchitis and H9N2 avian influenza viruses.
2.7. Statistical analysis
Data were analysed by ANOVA method followed by Tukey's multiple comparison test or Kruskal–Wallis followed by Dunn's multiple comparison test where appropriate. Data related to virus detection period and mortality were analysed by Fisher's exact test. The condition p < 0.05 was considered the level of significance for all comparisons. Data analysis and preparation of graphs were performed by GraphPad Prism 6 software.
3. RESULTS
3.1. Performance parameters, severity of clinical signs and mortality rate
Table 2 summarizes data related to body weight, FCR and severity of clinical signs in different groups. Birds in PC and AB groups showed a slight decrease in body weight as compared to NC group (p > 0.05) at the end of the experiment. FCR was lower in NC group as compared to birds in PC and AB groups. The severity of clinical scores was significantly higher in birds of PC and AB groups as compared to NC birds on days 3, 6 and 9 pc. On 12th day, the severity of clinical signs was statistically the same among all groups (p > 0.05). At none of the sampling time points, the administration of enrofloxacin appreciably affected the severity of clinical signs as compared to PC birds (p > 0.05). No mortality was observed in birds of the NC group during the experiment. Birds in PC group showed slightly higher mortality than AB‐treated birds (p > 0.05).
TABLE 2.
Performance parameters, clinical scores and mortality rate of different groups.
| Groups | Body weight (g) | FCR | Clinical signs score | Mortality | ||||
|---|---|---|---|---|---|---|---|---|
| Day 25 | Day 37 | Days post‐challenge | ||||||
| 3rd | 6th | 9th | 12th | |||||
| NC | 1100 ± 45a | 2240 ± 111a | 1.98 | 1 ± 0a | 1 ± 0a | 1 ± 0a | 1 ± 0a | 0/15a |
| PC | 1094 ± 34a | 2111 ± 118a | 2.18 | 2.7 ± 0.67b | 3.2 ± 0.42b | 2.8 ± 0.79b | 2 ± 0.67a | 3/15b |
| AB | 1100 ± 45a | 2181 ± 115a | 2.15 | 2.7 ± 0.67b | 3.2 ± 0.42b | 2.8 ± 0.79b | 2.1 ± 0.74a | 2/15b |
Note: Data for parameters other than FCR and mortality rate are presented as mean ± SD. Different superscript letters show significant difference in a column (p < 0.05). NC: negative control (no treatment); PC: (positive control) birds that challenged with H9N2 and infectious bronchitis viruses; AB: challenged birds that received enrofloxacin in drinking water after appearance of symptoms for 5 consecutive days.
3.2. Gross pathological findings
Necropsy findings of birds that died during the course of experiment mostly included mild‐to‐moderate tracheal hyperaemia, presence of casts in syrinx, mild‐to‐moderate inflammation of kidney and spleen, air sacculitis and pleuropneumonia. These lesions were not scored and only addressed qualitatively. No euthanized bird showed the presence of tracheal casts or casts in syrinx. The scores of gross lesions in each group are summarized in Table 3. The severity of tracheal lesions, including hyperaemia and the presence of exudative secretions in PC and AB groups, was reduced by day 9 of the experiment, whereas no change in congestion of lungs was observed until day 12 pc. The most profound effect of AB administration was the reduction of pleuropneumonia severity score on day 12 pc as compared to PC group (p < 0.05). Although the severity of air sacculitis in birds of AB group was lower than PC birds on days 6, 9 and 12 pc, the difference was not statistically significant (p > 0.05).
TABLE 3.
Gross pathological scores (median) of lesion in respiratory tract of different groups.
| Gross pathological scores | ||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Days post‐challenge | ||||||||||||||||||||||||||||
| Groups | 3rd | 6th | 9th | 12th | ||||||||||||||||||||||||
| TH | TE | TC | CC | LC | PP | AS | TH | TE | TC | CC | LC | PP | AS | TH | TE | TC | CC | LC | PP | AS | TH | TE | TC | CC | LC | PP | AS | |
| NC | 0a | 0a | 0a | 0a | 0a | 0a | 0a | 0a | 0a | 0a | 0a | 0a | 0a | 0a | 0a | 0a | 0a | 0a | 0a | 0a | 0a | 0a | 0a | 0a | 0a | 0a | 0a | 0a |
| PC | 2b | 2b | 0a | 0a | 1b | 0a | 1b | 2b | 1ab | 0a | 0a | 1b | 1b | 2b | 1b | 0a | 0a | 0a | 1b | 1b | 2b | 1b | 0a | 0a | 0a | 1b | 3b | 3b |
| AB | 2b | 2b | 0a | 0a | 1b | 0a | 1b | 2b | 2b | 0a | 0a | 1b | 1b | 1ab | 1b | 0a | 0a | 0a | 1b | 1b | 1ab | 1b | 0a | 0a | 0a | 1b | 0a | 1ab |
Note: Different superscript letters show significant difference in a column (p < 0.05). NC: negative control (no treatment); PC: (positive control) birds that challenged with H9N2 and infectious bronchitis viruses; AB: challenged birds that received enrofloxacin in drinking water after appearance of symptoms for 5 consecutive days.
Abbreviations: AS, air sacculitis; CC, casts in syrinx; LC, lung congestion; PP, pleuropneumonia; TC, tracheal casts; TE, tracheal exudates; TH, tracheal hyperaemia.
3.3. Histopathological findings
No lesion was observed in the trachea of NC group (Figure 1a). Histopathological changes, including tracheal epithelial changes, lymphocytes infiltration, loss of cilia and oedema in lamina propria, were observed in birds of PC (Figure 1b) and AB groups (Figure 1c) from day 3 pc and fulminated on 9th day. Consistent with clinical signs, the severity of histopathological changes in these groups decreased in 12th day pc, whereas the scores remained significantly higher than NC group (p < 0.05). At all sampling time points, the administration of AB was not associated with a significant amelioration of histopathological changes as compared to PC birds (p > 0.05). Data are shown in Table 4.
FIGURE 1.
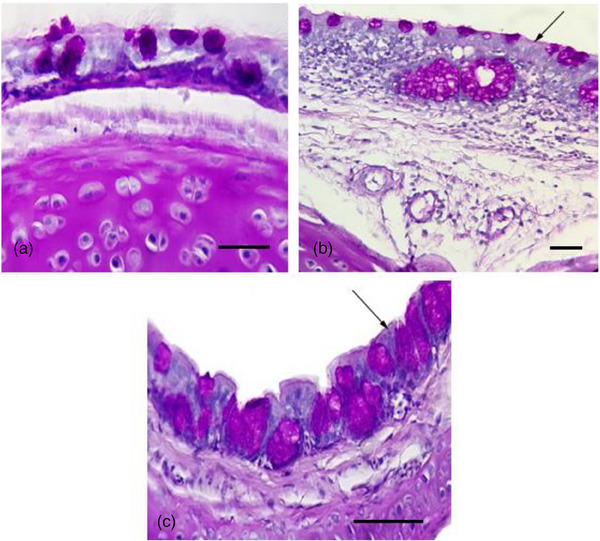
Representative photomicrographs of tracheal tissue in negative control (a), positive control (b) and enrofloxacin‐treated (c) birds on day 3 post‐challenge (periodic acid–Schiff [PAS] staining, 40×; scale bar: 20 µm). Lymphocytes infiltration, loss of cilia (arrows) and oedema in lamina propria are present in birds of positive control and enrofloxacin‐treated groups.
TABLE 4.
Histopathological lesion scores (mean ± SD) of trachea in birds of different groups.
| Groups | Histopathological lesion score | |||
|---|---|---|---|---|
| Days post‐challenge | ||||
| 3rd | 6th | 9th | 12th | |
| NC | 1.1 ± 0.18a | 1.1 ± 0.18a | 1 ± 0a | 1 ± 0a |
| PC | 2.4 ± 0.43b | 3.13 ± 0.56b | 3.73 ± 0.28b | 2.7 ± 0.64b |
| AB | 2.4 ± 0.55 b | 3.13 ± 0.38b | 3.54 ± 0.38b | 2.6 ± 0.43b |
Note: Different superscript letters show significant difference in a column (p < 0.05). NC: negative control (no treatment); PC: (positive control) birds that challenged with H9N2 and infectious bronchitis viruses; AB: challenged birds that received enrofloxacin in drinking water after the appearance of symptoms for 5 consecutive days.
3.4. Number of goblet cells
The numbers of goblet cells counted in tracheal samples obtained on days 3 and 6 pc are shown in Figure 2. On day 3 pc, a slight insignificant increase was observed in the number of goblet cells of PC group as compared to NC birds, whereas birds in AB group had a significantly higher number of goblet cells as compared to NC (p < 0.01) but not PC group (p > 0.05). On day 6 pc, the number of goblet cells was significantly higher in tracheal samples of PC and AB groups as compared to NC birds (p < 0.0001) and administration of AB increased this parameter as compared to PC group (p < 0.05).
FIGURE 2.
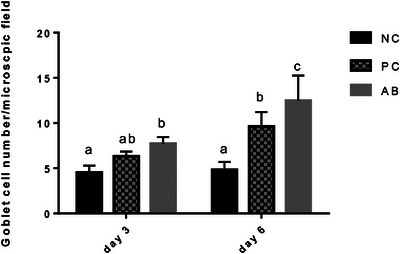
The number of goblet cells/microscopic field (mean and SD) of periodic acid–Schiff (PAS)‐stained tracheal samples from birds in different groups on days 3 and 6 post‐challenge with H9N2 and infectious bronchitis viruses. Different superscript letters show significant difference among columns in one sampling time point (p < 0.05).
3.5. Ciliostasis score
On both sampling time points, the ciliary activity in tracheal rings of birds in PC and AB groups was reduced which was manifested by significantly higher ciliostasis scores compared to NC birds (p < 0.0001). Administration of enrofloxacin did not significantly affect ciliary activity as compared to PC birds (p > 0.05 for both sampling time points). Data are shown in Figure 3.
FIGURE 3.
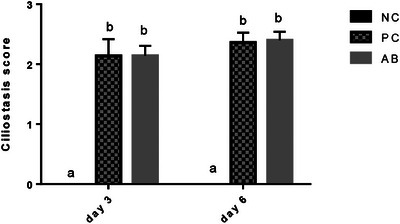
The ciliostasis scores (mean and SD) of tracheal samples from birds in different groups on days 3 and 6 post‐challenge with H9N2 and infectious bronchitis viruses. Different superscript letters show significant difference among columns in one sampling time point (p < 0.05).
3.6. Virus detection period
As previously stated, samples from trachea and caecal tonsils of five birds from different groups were collected on days 3, 6, 9 and 12 pc, and virus detection was performed by duplex RT‐PCR (Figure 4).
FIGURE 4.
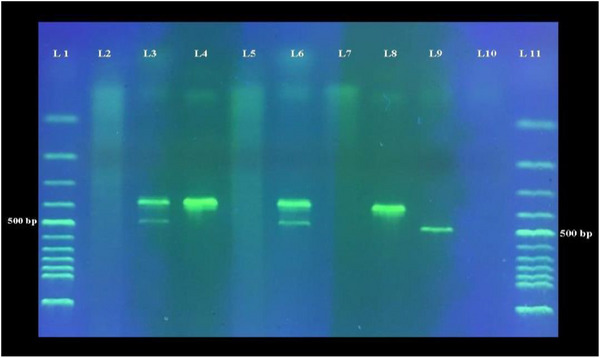
Gel electrophoresis of duplex RT‐PCR products. The 387 and 488 bp products are related to infectious bronchitis and H9N2 viruses, respectively. Lanes 1 and 11 are 100 bp marker, and lanes 2–7 are related to samples obtained from birds challenged with viruses. Lane 8 is positive control for infectious bronchitis virus, and lane 9 is positive control for H9 gene of H9N2 virus. Lane 10 is negative control.
The results related to virus detection on different days are shown in Table 5. None of the samples which were collected from NC birds contained H9N2 or IB viruses. For H9N2, virus was detected in tracheal (2/5) and caecal samples (1/5) of PC group from day 3 pc. The highest level of virus detection was on day 6 pc in this group (1/5 in tracheal samples and 3/5 in caecal tonsil samples). All samples were negative on day 12 pc. Like PC group, virus positive samples (2/5 in trachea and 1/5 in caecal tonsils) were detected in birds from AB group from day 3 pc, and the highest levels of positive samples were detected on day 6 (2/5 and 4/5 in tracheal and caecal samples, respectively). For IB virus, 4/5 of tracheal and caecal tonsil samples were positive on day 3 pc in both PC and AB groups. All tracheal samples and 4/5 of caecal tonsil samples were virus positive on days 6 and 9 pc in these groups. On day 12, 3/5 of both types of samples remained positive in PC and AB groups. No significant difference was observed in the positivity ratio of samples between PC and AB groups for both viruses (p > 0.05).
TABLE 5.
The fraction of H9N2 or IB virus positive samples to total samples obtained from trachea and caecal tonsils of birds in different days post‐challenge.
| Day post‐challenge | Tissue sample | Groups | |||||
|---|---|---|---|---|---|---|---|
| H9N2 | IB | ||||||
| NC | PC | AB | NC | PC | AB | ||
| 3 | Trachea | 0/5 | 2/5 | 2/5 | 0/5 | 4/5 | 4/5 |
| Caecal tonsil | 0/5 | 1/5 | 1/5 | 0/5 | 4/5 | 4/5 | |
| 6 | Trachea | 0/5 | 1/5 | 2/5 | 0/5 | 5/5 | 5/5 |
| Caecal tonsil | 0/5 | 3/5 | 4/5 | 0/5 | 4/5 | 4/5 | |
| 9 | Trachea | 0/5 | 0/5 | 0/5 | 0/5 | 5/5 | 5/5 |
| Caecal tonsil | 0/5 | 1/5 | 1/5 | 0/5 | 4/5 | 4/5 | |
| 12 | Trachea | 0/5 | 0/5 | 0/5 | 0/5 | 3/5 | 3/5 |
| Caecal tonsil | 0/5 | 0/5 | 1/5 | 0/5 | 3/5 | 3/5 | |
Note: No significant difference was observed between positive control (PC) and enrofloxacin‐treated (AB) birds.
Abbreviation: NC, negative control.
4. DISCUSSION
In a parallel controlled experimental study, we evaluated the effect of enrofloxacin as a broad‐spectrum antibacterial agent on the clinical outcome and mucociliary activity of respiratory system in broilers challenged with H9N2 and IB viruses. The results showed that except for the severity of pleuropneumonia on day 12 pc, administration of this AB was not associated with significant improvements in performance, mortality or clinical outcome of birds; neither it changed virus detection period nor the number of virus positive birds.
As previously stated, pleuropneumonia was observed in birds of PC and AB groups from day 6 pc. The severity of this lesion was most profound on day 12 pc in PC group, whereas birds in AB group showed significantly lower severity score. The presence of this gross pathological sign can be related to the complication of the disease by secondary bacterial infections like mycoplasma and E. coli. It has been clearly shown that in addition to immunosuppression (Yehia et al., 2023), H9N2 AIV infection increases the expression of inflammatory cytokines and promotes the proliferation and migration of proteobacteria, especially E. coli from chicken intestines due to damage to mucous membranes and tight junctions (Li et al.,2018 ) Complication of H9N2 infections in broilers with E. coli results in exacerbation of pneumonia as compared to birds only challenged with H9N2 virus (Jaleel et al., 2017). Fluoroquinolones have broad antibacterial effects. Therefore, it seems that by preventing bacterial complication of the viral respiratory diseases, enrofloxacin has mitigated the severity of pneumonia in birds and consequently resulted in slightly lower mortality rate. Whether plausible effects of enrofloxacin on humoural or cell‐mediated immune responses are associated with this finding cannot be concluded from the present study.
In the present study, although we observed no change in ciliary activity, the number of tracheal goblet cells increased on day 6 pc by antibacterial administration. Although avian respiratory tract has considerable species‐specific characteristics, like mammals, nonspecific immune mechanisms, such as aerodynamic filtration, mucociliary clearance and antimicrobial substances, play a pivotal role as the first line of defence in the respiratory tract of avian species (Sutton et al., 2018). The mucociliary system as a part of innate immune mechanism has an important role in preventing the entrance of foreign agents including pathogens to lower parts of respiratory tract. Respiratory mucus secreted by goblet cells traps pathogens entering the airways, and concerted cilia movements propel them outwards (Adivitiya et al., 2021).
Tracheal ciliostasis is observed in chickens with IB, and the degree of ciliostasis determines the degree of tracheal damage following IBV infection (Tarpey et al., 2006). In a study by Petersen et al. (2012), all tested LPAI viruses, including H9N2, were strong inducers of apoptosis upon infection in tracheal organ culture of chickens which resulted in ciliostasis. Consistently, we observed ciliostasis in tracheal rings of chickens challenged with H9N2/IB viruses which was not ameliorated by administration of enrofloxacin.
It has been demonstrated that IBV results in the loss of goblet cells in the tracheal mucosa of chickens (Nakamura et al., 1991); on the contrary, Sun et al. (2019) showed that H9N2 viruses induce a severe increase of goblet cells in the trachea of infected chickens. Therefore, the increased number of tracheal goblet cells which was observed from day 3 in infected groups of our study can be related to H9N2 but not IB infection.
Administration of enrofloxacin could not ameliorate the severity of tracheal lesions in histopathological evaluation, whereas it increased the number of goblet cells in tracheal mucosa on day 6 pc.
Sialic acids (SAs) linked to galactose (Gal) in α2,3‐ and α2,6‐configurations are the receptors for avian and human influenza viruses, respectively. In a study by Shen et al. (2011), it was demonstrated that chicken tracheal ciliated cells express α2,3‐linked SA, whereas goblet cells mainly express α2,6‐linked SA. Mucus, which is mainly composed of goblet cell‐secreted mucins, not only entraps foreign pathogens and particles but also acts as a reservoir of host‐protective molecules (Adivitiya et al., 2021). Therefore, the increased number of goblet cells which was observed due to enrofloxacin administration in AB group may help in entrapping and defence by possible increase in mucin secretion. This increasing effect of enrofloxacin on tracheal goblet cell number should be considered conservatively, as muco‐obstruction can be detrimental especially with regard to the fact that we did not find the improved activity of cilia or amelioration of histopathological lesions of trachea due to AB administration.
Altogether, the administration of enrofloxacin as a broad‐spectrum antibacterial agent to broilers challenged with H9N2/IB viruses was not associated with appreciable positive effects on most of the parameters which were evaluated in this study, including mortality, clinical signs, histopathological lesions and virus detection period in trachea and caecal tonsil. Although AB‐treated birds showed less severe pleuropneumonia on day 12 pc, possibly due to protection against secondary bacterial infections, the effect of enrofloxacin on mucociliary system was inconclusive.
In conclusion, we found a weak positive effect of enrofloxacin administration in H9N2/IB‐infected chickens. Considering the risks of AB treatment in broiler chickens, the results of this small‐scale study do not encourage the benefit of enrofloxacin use in these viral diseases, although performing future risk–benefit balance studies on a large number of birds and for longer periods following infection is suggested.
AUTHOR CONTRIBUTIONS
Conceptualization; investigation; methodology; writing – review and editing: Mohammad Abbasnia. Conceptualization; formal analysis; funding acquisition; methodology; project administration; supervision; writing – review and editing: Najmeh Mosleh. Conceptualization; funding acquisition; methodology; project administration; supervision; writing – review and editing: Habibollah Dadras. Conceptualization; formal analysis; writing – original draft: Tahoora Shomali.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
FUNDING INFORMATION
Shiraz University
ETHICS STATEMENT
Procedures used in the present study are approved by institutional ethical committee (95GCU2M163723) and are in accordance with Directive 2010/63/EU.
PEER REVIEW
The peer review history for this article is available at https://www.webofscience.com/api/gateway/wos/peer-review/10.1002/vms3.1390.
Abbasnia, M. , Mosleh, N. , Dadras, H. , & Shomali, T. (2024). Effect of enrofloxacin on clinical parameters and mucociliary system of broilers challenged with H9N2 avian influenza/infectious bronchitis viruses. Veterinary Medicine and Science, 10, e1390. 10.1002/vms3.1390
DATA AVAILABILITY STATEMENT
Data available on request from the authors.
REFERENCES
- Abbasnia, M. , Mosleh, N. , Dadras, H. , Rezaeinzadeh, G. H. , & Boroomand, Z. (2020). Effect of different herbal preparations on experimental viral respiratory complex of broilers: clinical, pathological and ciliary activity aspects. Journal of Herbmed Pharmacology, 9, 277–285. [Google Scholar]
- Adivitiya, K. M. S. , Chakraborty, S. , Veleri, S. , & Kateriya, S. (2021). Mucociliary respiratory epithelium integrity in molecular defense and susceptibility to pulmonary viral infections. Biology (Basel), 10(2), 95. 10.3390/biology10020095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amanollahi, R. , Asasi, K. , Abdi‐Hachesoo, B. , Ahmadi, N. , & Mohammadi, A. (2021). Effect of infectious bronchitis and Newcastle disease vaccines on experimental avian influenza infection (H9N2) in broiler chickens. Bulgarian Journal of Veterinary Medicine, 24, 574–585. [Google Scholar]
- Bano, S. , Naeem, K. , & Malik, S. A. (2003). Evaluation of pathogenic potential of avian influenza virus serotype H9N2 in chickens. Avian Diseases, 47(Suppl 3), 817–822. 10.1637/0005-2086-47.s3.817 [DOI] [PubMed] [Google Scholar]
- Belkasmi, S. F. Z. , Fellahi, S. , Touzani, C. D. , Faraji, F. Z. , Maaroufi, I. , Delverdier, M. , Guérin, J. L. , Fihri, O. F. , El Houadfi, M. , & Ducatez, M. F. (2020). Co‐infections of chickens with avian influenza virus H9N2 and Moroccan Italy 02 infectious bronchitis virus: Effect on pathogenesis and protection conferred by different vaccination programmes. Avian Pathology, 49(1), 21–28. [DOI] [PubMed] [Google Scholar]
- Ennaji, Y. , Khataby, K. , & Ennaji, M. M. (2020). Infectious bronchitis virus in poultry: Molecular epidemiology and factors leading to the emergence and reemergence of novel strains of infectious bronchitis virus. Emerging and Reemerging Viral Pathogens, 2, 31–44. 10.1016/B978-0-12-814966-9.00003-2 [DOI] [Google Scholar]
- Ghalyanchi‐Langeroudi, A. , Karimi, V. , Jannat, A. , Hashemzadeh, M. , Fallah, M. H. , Gholami, F. , Zabihi, M. T. , & Heidarzadeh, M. (2015). Genotyping of infectious bronchitis viruses in the East of Iran, 2015. Iranian Journal of Virology, 9(2), 31–35. [Google Scholar]
- Gray, P. , Jenner, R. , Norris, J. , Page, S. , & Browning, G. (2021). Australian Veterinary Association Ltd and Animal Medicines Australia. Antimicrobial prescribing guidelines for poultry. Australian Veterinary Journal, 99(6), 181–235. 10.1111/avj.13034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan, K. E. , Ali, A. , Shany, S. A. S. , & El‐Kady, M. F. (2017). Experimental co‐infection of infectious bronchitis and low pathogenic avian influenza H9N2 viruses in commercial broiler chickens. Research in Veterinary Science, 115, 356–362. 10.1016/j.rvsc.2017.06.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackwood, M. W. , Rosenbloom, R. , Petteruti, M. , Hilt, D. A. , McCall, A. W. , & Williams, S. M. (2010). Avian coronavirus infectious bronchitis virus susceptibility to botanical oleoresins and essential oils in vitro and in vivo. Virus Research, 149(1), 86–94. 10.1016/j.virusres.2010.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackwood, M. W. , Jordan, B. J. , Roh, H. J. , Hilt, D. A. , & Williams, S. M. (2015). Evaluating protection against infectious bronchitis virus by clinical signs, ciliostasis, challenge virus detection, and histopathology. Avian Diseases, 59(3), 368–374. 10.1637/11026-012415-Reg.1 [DOI] [PubMed] [Google Scholar]
- Jaleel, S. , Younus, M. , Idrees, A. , Arshad, M. , Khan, A. U. , Ehtisham‐ul‐Haque, S. , Zaheer, M. I. , Tanweer, M. , Towakal, F. , Munibullah, Tipu, M. Y. , Sohail, M. L. , & Umar, S. (2017). Pathological alterations in respiratory system during co‐infection with low pathogenic avian influenza virus (H9N2) and Escherichia coli in broiler chickens. Journal of Veterinary Research, 61(3), 253–258. 10.1515/jvetres-2017-0035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalifeh, M. S. , Amawi, M. M. , Abu‐Basha, E. A. , & Yonis, I. B. (2009). Assessment of humoral and cellular‐mediated immune response in chickens treated with tilmicosin, florfenicol, or enrofloxacin at the time of Newcastle disease vaccination. Poultry Science, 88(10), 2118–2124. 10.3382/ps.2009-00215 [DOI] [PubMed] [Google Scholar]
- Lee, M. S. , Chang, P. C. , Shien, J. H. , Cheng, M. C. , & Sheih, H. K. (2001). Identification and subtyping of avian influenza viruses by reverse transcription‐PCR. Journal of Virological Methods, 97, 13–22. [DOI] [PubMed] [Google Scholar]
- Li, H. , Liu, X. , Chen, F. , Zuo, K. , Wu, C. , Yan, Y. , Chen, W. , Lin, W. , & Xie, Q. (2018). Avian influenza virus subtype H9N2 affects intestinal microbiota, barrier structure injury, and inflammatory intestinal disease in the chicken ileum. Viruses, 10, 270. 10.3390/v10050270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnusson, U. (2021). How to use antibiotics effectively and responsibly in poultry production for the sake of human and animal health. Budapest FAO, http://www.fao.org/3/cb4157en/cb4157en.pdf [Google Scholar]
- Nakamura, K. , Cook, J. K. , Otsuki, K. , Huggins, M. B. , & Frazier, J. A. (1991). Comparative study of respiratory lesions in two chicken lines of different susceptibility infected with infectious bronchitis virus: Histology, ultrastructure and immunohistochemistry. Avian Pathology, 20(2), 241–257. 10.1080/03079459108418761 [DOI] [PubMed] [Google Scholar]
- Petersen, H. , Matrosovich, M. , Pleschka, S. , & Rautenschlein, S. (2012). Replication and adaptive mutations of low pathogenic avian influenza viruses in tracheal organ cultures of different avian species. PLoS ONE, 7(8), e42260. 10.1371/journal.pone.0042260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen, C. I. , Wang, C. H. , Shen, S. C. , Lee, H. C. , Liao, J. W. , & Su, H. L. (2011). The infection of chicken tracheal epithelial cells with a H6N1 avian influenza virus. PLoS ONE, 6(5), e18894. 10.1371/journal.pone.0018894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu, T. , Shimizu, S. , Hattori, R. , Gabazza, E. C. , & Majima, Y. (2003). In vivo and in vitro effects of macrolide antibiotics on mucus secretion in airway epithelial cells. American Journal of Respiratory and Critical Care Medicine, 168(5), 581–587. 10.1164/rccm.200212-1437OC [DOI] [PubMed] [Google Scholar]
- Snyder, D. B. , Marquardt, W. W. , & Kadavil, S. K. (1983). Ciliary activity: A criterion for associating resistance to infectious bronchitis virus infection with ELISA antibody titer. Avian Diseases, 27(2), 485–490. [PubMed] [Google Scholar]
- Sun, H. , Wang, K. , Yao, W. , Liu, Q. , Yang, J. , Teng, Q. , Li, X. , Li, Z. , & Chen, H. (2019). H9N2 viruses isolated from mammals replicated in mice at higher levels than avian‐origin viruses. Frontiers in Microbiology, 10, 416. 10.3389/fmicb.2019.00416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton, K. , Costa, T. , Alber, A. , Bryson, K. , Borowska, D. , Balic, A. , Kaiser, P. , Stevens, M. , & Vervelde, L. (2018). Visualisation and characterisation of mononuclear phagocytes in the chicken respiratory tract using CSF1R‐transgenic chickens. Veterinary Research, 49(1), 104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swayne, D. E. (2021). https://www.msdvetmanual.com/poultry/avian‐influenza/avian‐influenza
- Takemura, H. , Tamaoki, J. , Chiyotani, A. , & Konno, K. (1996). Effect of ciprofloxacin on ciliary motility of rabbit airway epithelium. Journal of Antimicrobial Chemotherapy, 38(1), 139–143. 10.1093/jac/38.1.139 [DOI] [PubMed] [Google Scholar]
- Takeyama, K. , Tamaoki, J. , Chiyotani, A. , Tagaya, E. , & Konno, K. (1993). Effect of macrolide antibiotics on ciliary motility in rabbit airway epithelium in‐vitro. Journal of Pharmacy and Pharmacology, 45(8), 756–758. 10.1111/j.2042-7158.1993.tb07104.x [DOI] [PubMed] [Google Scholar]
- Tarpey, I. , Orbell, S. J. , Britton, P. , Casais, R. , Hodgson, T. , Lin, F. , Hogan, E. , & Cavanagh, D. (2006). Safety and efficacy of an infectious bronchitis virus used for chicken embryo vaccination. Vaccine, 24(47–48), 6830–6838. 10.1016/j.vaccine.2006.06.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehia, N. , Salem, H. M. , Mahmmod, Y. , Said, D. , Samir, M. , Mawgod, S. A. , Sorour, H. K. , AbdelRahman, M. A. A. , Selim, S. , Saad, A. M. , El‐Saadony, M. T. , El‐Meihy, R. M. , Abd El‐Hack, M. E. , El‐Tarabily, K. A. , & Zanaty, A. M. (2023). Common viral and bacterial avian respiratory infections: An updated review. Poultry Science, 102(5), 102553. 10.1016/j.psj.2023.102553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, X. , Zhao, Q. , Ci, X. , Chen, S. , Chen, L. , Lian, J. , Xie, Z. , Ye, Y. , Lv, H. , Li, H. , Lin, W. , Zhang, H. , & Xie, Q. (2020). Effect of baicalin on bacterial secondary infection and inflammation caused by H9N2 AIV infection in chickens. BioMed Research International, 2020, 2524314. 10.1155/2020/2524314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Y. , Xu, Z. , & Cao, Y. (2021). Host antiviral responses against avian infectious bronchitis virus (IBV): Focus on innate immunity. Viruses, 13(9), 1698. 10.3390/v13091698 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data available on request from the authors.


