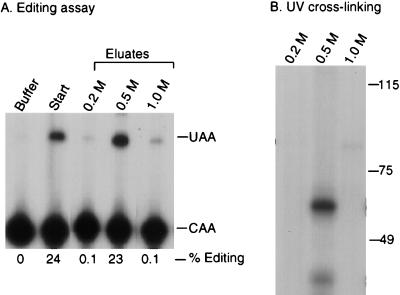
FIG. 3.
UV-crosslinking of a 65-kDa protein correlates with complementing activity. (A) RNA affinity chromatography was performed as described in Materials and Methods. Editing assays were performed on the 0.2, 0.5, or 1 M-salt-eluted fractions from the wild-type RNA affinity resin. Editing assays were performed as described in the legend to Fig. 1. (B) UV cross-linking was performed with the salt-eluted fractions. Proteins were incubated with 32P-labeled wild-type RNA and exposed to short-wave UV light for 10 min. After treatment with RNase A, samples were analyzed by SDS–7.5% PAGE and autoradiography. Molecular size standards (in kilodaltons) are indicated on the right of each panel. The figure was generated by using ScanWizard 1.05 (Microtek) and Adobe Photoshop 3.05.