Abstract
Background:
Whether initial invasive management in older versus younger adults with chronic coronary disease (CCD) and moderate or severe ischemia improves health status or clinical outcomes is unknown.
Objectives:
Examine the impact of age on health status and clinical outcomes with invasive versus conservative management in the ISCHEMIA trial.
Methods:
One-year angina-specific health status was assessed with the 7-item Seattle Angina Questionnaire (SAQ; score range 0–100; higher=better health status). Cox proportional hazards models estimated the treatment effect of invasive versus conservative management as a function of age on the composite clinical outcome of cardiovascular death, myocardial infarction, or hospitalization for resuscitated cardiac arrest, unstable angina, or heart failure.
Results:
Among 4617 participants, 2239 (48.5%) were <65, 1713 (37.1%) were 65–74, and 665 (14.4%) were ≥75 years old. Baseline SAQ Summary Scores were lower in participants <65 years old. Fully adjusted differences in 1-year SAQ Summary Scores (invasive minus conservative) were 4.90 (95% CI 3.56, 6.24) at age 55, 3.48 (95% CI 2.40, 4.57) at age 65, and 2.13 (95% CI 0.75, 3.51) at age 75 (interaction P=0.008). Improvement in SAQ Angina Frequency was less dependent on age (interaction P=0.07). There were no age differences between invasive versus conservative management on the composite clinical outcome (interaction P=0.29).
Conclusions:
Older patients with CCD and moderate or severe ischemia had consistent improvement in angina frequency but less improvement in angina-related health status with invasive management compared with younger patients. Invasive management was not associated with improved clinical outcomes in older or younger patients.
Keywords: coronary artery disease, quality of life, older adults
CONDENSED ABSTRACT
In this secondary analysis of the ISCHEMIA trial, we compared angina-related health status and clinical outcomes with an invasive vs conservative strategy as a function of age in adults with chronic coronary disease (CCD) and moderate or severe ischemia. An invasive strategy resulted in consistently better angina relief at 12-months in all age groups compared with a conservative strategy, but older adults had less improvement in angina-related health status compared with younger adults. Both treatment approaches resulted in similar 4-year clinical event rates, independent of age. This knowledge can help inform shared decision-making between clinicians and older adults with CCD.
INTRODUCTION
The decision to perform revascularization in older adults with chronic coronary disease (CCD) can be challenging, as they are likely to have more complex coronary artery disease and more comorbidities1,2, leading to increased risks of peri-procedural death, acute kidney injury, and major bleeding after percutaneous coronary intervention (PCI)3–5 or coronary artery bypass grafting (CABG).6 Based on evidence suggesting that revascularization does not reduce clinical events when added to guideline-directed medical therapy (GDMT) in patients with CCD without extensive CAD or heart failure7–9, current guidelines recommend GDMT as the initial treatment strategy in many older adults with CCD.1 However, revascularization may be offered more readily if there is a pressing need to improve patients’ symptoms, physical function, and quality of life (QOL) or for select anatomic findings such as left main disease. Particularly in older adults, symptom burden and functional independence may be more important than longevity when considering treatment options.10,11 As such, defining angina relief and health status benefits is important in counseling older adults about their treatment options, given their higher risk of peri-procedural complications.
To better understand the health status and clinical outcomes in older adults with CCD, we leveraged the ISCHEMIA trial (International Study of Comparative Health Effectiveness with Medical and Invasive Approaches), which compared invasive treatment plus GDMT versus a conservative strategy of GDMT alone in participants with CCD and moderate or severe ischemia. The main findings from ISCHEMIA were that clinical event rates were similar between treatment groups but an initial invasive strategy resulted in greater improvements in angina-related health status compared with an initial conservative strategy.12,13 To better elucidate the treatment benefits in older adults with CCD and inform shared decision-making in this rapidly growing population14, we investigated the effectiveness of an invasive strategy on health status outcomes and clinical event rates across the age spectrum. As ISCHEMIA enrolled 693 participants ≥75 years old, more than any previous strategy trial for management of CCD,8,9 it offers a unique opportunity to explore this important question.
METHODS
ISCHEMIA Trial
The design and results of the ISCHEMIA trial have been published previously.12,15 Briefly, ISCHEMIA was a randomized, parallel assignment, unblinded trial that included 5,179 participants with CCD and moderate or severe ischemia on stress testing. Participants were randomized 1:1 to a routine invasive strategy (GDMT with angiography and revascularization, if feasible) or conservative strategy (GDMT only, with angiography and revascularization reserved for failure of medical therapy). The study protocol was approved by the New York University Grossman School of Medicine (the study coordinating site) and by the institutional review boards and ethics committees at each participating site. All participants provided written informed consent.
Study Population and Definition of Older Adult
To characterize both the health status and clinical outcomes of ISCHEMIA participants across the spectrum of age, we included only those with complete baseline health status data and at least one health status assessment during the trial follow-up period, in line with the main ISCHEMIA health status analysis.13 We categorized participants into three age groups based on age at the time of study enrollment: <65 years old, 65–74 years old, and ≥75 years old.
Health Status Outcomes
Angina-related health status was measured with the 7-item Seattle Angina Questionnaire (SAQ)16 before randomization and at 1.5, 3, 6 months, and then every 6 months thereafter until the trial was completed. Although health status data were collected through 78 months, we focused the health status analyses on 12-month outcomes as this represents a clinically relevant time frame for shared decision-making with patients. The SAQ captures the frequency of angina (SAQ Angina Frequency), the effect of angina on physical functioning (SAQ Physical Limitation), and angina-related QOL (SAQ Quality of Life) over the prior 4 weeks. Scores for each domain are averaged to create a SAQ Summary Score. The SAQ has been shown to be highly valid, reliable, and sensitive to changes in clinical status,16–18 and predictive of future death, acute coronary syndrome, and healthcare costs.19,20 Scores range from 0 to 100, with higher scores signifying less angina, better physical functioning, and higher QOL, with a 5-point difference in scores considered clinically important.21 To facilitate interpretability, scores for each domain can be categorized into scores of 0–24 (very poor to poor health status), 25–49 (poor to fair), 50–75 (fair to good) and 76–100 (good to excellent). For SAQ Angina Frequency, score ranges of 0–30, 31–60, 61–99, and 100 represent daily, weekly, monthly, and no angina, respectively.22
General health status was measured with European Quality of Life- 5 Dimension Visual Analog Scale (EQ-5D VAS).23 Scores range from 0 to 100, with a score of zero indicating the worst possible health and a score of 100 indicating perfect health. Severity of dyspnea was assessed with the Rose Dyspnea Scale (RDS), which assesses whether dyspnea is present with four common physical activities.24 One point is assigned to each activity where dyspnea occurs, with scores of 0 indicating no dyspnea and scores of 4 indicating significant limitations due to dyspnea.
Clinical Outcomes
The primary clinical outcome of the ISCHEMIA trial was the composite of cardiovascular death, non-fatal myocardial infarction (MI), or hospitalization for heart failure, unstable angina, or resuscitated cardiac arrest. Other outcomes included the composite of cardiovascular death and non-fatal MI, all-cause death, stroke, and the individual events comprising the primary composite outcome. The definitions of non-procedural MI were based on the Third Universal Definition of MI for types 1, 2, 4b, and 4c, but higher biomarker thresholds were pre-specified for defining procedural MI (MI types 4a and 5).12,25
Statistical Analysis
For descriptive purposes, continuous variables are summarized as mean ± standard deviation or median (interquartile range), and categorical variables as percentages, stratified by the 3 selected age groups (<65, 65–74, ≥75 years old).
To explore the association of age with health status over 12 months, we first examined unadjusted SAQ Summary Score, SAQ Angina Frequency, SAQ Physical Limitation, SAQ Quality of Life, EQ-5D VAS, and RDS by treatment strategy and age category at baseline, 3-months, 6-months, and 12-months. We also examined the proportion of participants who were angina-free (SAQ Angina Frequency = 100) and dyspnea-free (RDS = 0) at each follow-up period. We then used multivariable linear regression models to estimate the difference in health status outcomes between invasive and conservative strategies for each health status outcome at 12 months, including age as a continuous variable, treatment group, an age-by-treatment interaction, and adjusting for geographic region, sex, smoking, body mass index, and baseline scores for each health status outcome with restricted cubic splines to allow for non-linear effects at the 5th, 35th, 65th, and 95th percentiles of the age distribution. The number of knots was selected based on model AIC and visual inspection of the estimated curves. Age-related comorbidities were initially excluded from this model to avoid over-adjusting for the effect of age on treatment outcomes. We then developed a fully adjusted model to examine the impact of comorbidities on health status outcomes, that included the above covariates plus hypertension, degree of ischemia, number of diseased vessels, congestive heart failure, New York Heart Association class, diabetes, estimated glomerular filtration rate, myocardial infarction, prior PCI, prior CABG, peripheral artery disease, and stroke/transient ischemic attack. As a sensitivity analysis, because the health status benefit of revascularization was largest in participants with more baseline angina,13 we repeated the health status models excluding participants without baseline angina (SAQ Angina Frequency = 100).
Methods for handling missing covariate and health status data have been described elsewhere.12,13 To account for missing data, we used multiple imputation by chained equations.26 The imputation model included all baseline covariates, randomized treatment, an age-by-treatment interaction, and all available health status scores from baseline through 12 months. A total of 64 randomly imputed data sets were generated; the analyses described above were repeated on each data set and the results were pooled using Rubin’s method to obtain final model estimates.27 The resulting estimates were similar to those obtained on complete-data cases; only imputed-data results are reported here. Rates of missing health status data are presented in Supplemental Table S1.
To define the association of age with clinical events, we first fit Cox proportional hazards models for each outcome over 4 years of follow-up with age as a categorical variable (<65, 65–74, ≥74 years) and randomized treatment group. To examine whether there was heterogeneity of treatment benefit for clinical events, we then fit Cox proportional hazards models for each outcome with age as a continuous variable, treatment group, an age-by-treatment interaction, and adjusting for geographic region, sex, smoking, and BMI. We then developed a fully adjusted model that also included hypertension, degree of ischemia, number of diseased vessels, congestive heart failure, New York Heart Association class, diabetes, estimated glomerular filtration rate, myocardial infarction, prior PCI, prior CABG, peripheral artery disease, and stroke/transient ischemic attack. Results are presented as cause-specific hazard ratios (95% CI).
All statistics were performed with SAS version 9.4 (SAS Institute, Cary, NC), and R Statistical Software (R Foundation for Statistical Computing, Vienna, Austria). A p-value of <0.05 was considered statistically significant.
RESULTS
Of the 5179 participants who underwent randomization in the ISCHEMIA trial, we excluded 51 in the invasive arm and 30 in the conservative arm who did not have a baseline or any follow-up health status assessments, and 481 from five sites because of improper health status form collection, leaving 4617 participants for this analysis. There were no significant differences between included and excluded participants.13 Of included participants, median age was 65 years (IQR 59,71) with 2239 (48.5%) <65 years old, 1713 (37.1%) 65 to 74 years old, and 665 (14.4%) ≥75 years old. Baseline characteristics are presented by age group in Table 1 and by treatment group in Supplemental Table S2. Older adults were more likely to be White, female, and have cardiovascular comorbidities including hypertension, heart failure, valvular heart disease, atrial fibrillation, stroke, and peripheral vascular disease, but were less likely to have diabetes or be active smokers. Older adults more frequently had multiple morbidities (defined as ≥3 of the following: prior MI, revascularization, stroke, valvular heart disease, heart failure, atrial fibrillation, diabetes, smoking, hypertension, eGFR <60 mL/min/1.73 m2, or chronic lung disease). Compared with adults <65 years old and 65–74 years old, adults ≥75 years old at baseline were more likely to have an LDL-C <70mg/dL but less likely to be taking a high-intensity statin or have a SBP <140 mmHg.
Table 1.
Baseline clinical characteristics by age group.
| Age <65 | Age 65–74 | Age ≥75 | |
|---|---|---|---|
| (N=2,239) | (N=1,713) | (N=665) | |
|
| |||
| Age, years | 59 (53, 62) | 69 (67, 72) | 79 (77, 81) |
| Female sex | 501/2239 (22.4%) | 388/1713 (22.7%) | 183/665 (27.5%) |
| Race | |||
| White | 1431/2218 (64.5%) | 1347/1697 (79.4%) | 580/655 (88.5%) |
| Asian | 658/2218 (29.7%) | 265/1697 (15.6%) | 56/655 (8.5%) |
| Black | 110/2218 (5.0%) | 74/1697 (4.4%) | 16/655 (2.4%) |
| Prior myocardial infarction | 437/2233 (19.6%) | 376/1712 (22.0%) | 138/659 (20.9%) |
| Prior PCI | 452/2237 (20.2%) | 389/1712 (22.7%) | 175/664 (26.4%) |
| Prior CABG | 63/2239 (2.8%) | 90/1713 (5.3%) | 43/665 (6.5%) |
| Peripheral vascular disease | 61/2232 (2.7%) | 96/1709 (5.6%) | 42/665 (6.3%) |
| Prior stroke | 61/2239 (2.7%) | 59/1712 (3.4%) | 24/665 (3.6%) |
| Hypertension | 1579/2231 (70.8%) | 1389/1709 (81.3%) | 548/662 (82.8%) |
| Diabetes | 896/2239 (40.0%) | 734/1713 (42.8%) | 244/665 (36.7%) |
| Chronic lung disease | 99/2232 (4.4%) | 124/1711 (7.2%) | 65/663 (9.8%) |
| Heart failure | 174/2236 (7.8%) | 129/1712 (7.5%) | 72/665 (10.8%) |
| Valvular heart disease | 31/2132 (1.5%) | 42/1619 (2.6%) | 38/617 (6.2%) |
| Atrial fibrillation | 48/2237 (2.1%) | 92/1711 (5.4%) | 77/664 (11.6%) |
| Multimorbidity* | 267/2208 (12.1%) | 295/1691 (17.4%) | 152/650 (23.4%) |
| LVEF, % | 60 (55, 65) | 60 (55, 65) | 60 (55, 65) |
| Degree of ischemia | |||
| None | 99/2208 (4.5%) | 97/1693 (5.7%) | 51/662 (7.7%) |
| Mild | 161/2208 (7.3%) | 117/1693 (6.9%) | 53/662 (8.0%) |
| Moderate | 741/2208 (33.6%) | 656/1693 (38.7%) | 240/662 (36.3%) |
| Severe | 1207/2208 (54.7%) | 823/1693 (48.6%) | 318/662 (48.0%) |
| Number of diseased vessels (≥70%) by CCTA§ | |||
| 0 | 116/1137 (10.2%) | 128/817 (15.7%) | 52/262 (19.8%) |
| 1 | 483/1137 (42.5%) | 332/817 (40.6%) | 91/262 (34.7%) |
| 2 | 318/1137 (28.0%) | 220/817 (26.9%) | 75/262 (28.6%) |
| 3 | 220/1137 (19.3%) | 137/817 (16.8%) | 44/262 (16.8%) |
| Multivessel disease (≥70%) by CCTA | 668/1360 (49.1%) | 451/979 (46.1%) | 147/316 (46.5%) |
| Historyofangina | 2048/2239(91.5%) | 1473/1713(86.0%) | 571/665(85.9%) |
| New/worsening angina in the last 3 months | 616/2232(27.6%) | 414/1712(24.2%) | 178/661(26.9%) |
| SAQ-7 Summary Score | 71.8 ± 18.9 | 76.2 ± 18.2 | 76.4 ± 20.0 |
| SAQ-7 Angina Frequency | 79.0 ± 19.8 | 83.6 ± 18.9 | 84.2 ± 19.9 |
| Angina Frequency Category | |||
| Daily/weekly | 538 (24.0%) | 286 (16.7%) | 110 (16.6%) |
| Monthly | 1059 (47.3%) | 728 (42.6%) | 256 (38.6%) |
| None | 641 (28.6%) | 696 (40.7%) | 298 (44.9%) |
| SAQ-7 Physical Limitations | 79.4 ± 23.3 | 80.6 ± 22.8 | 75.8 ± 26.1 |
| SAQ-7 Quality of Life | 57.6 ± 26.0 | 64.8 ± 25.8 | 68.6 ± 26.9 |
| Rose Dyspnea Scale | 1.2 ± 1.3 | 1.1 ± 1.3 | 1.3 ± 1.4 |
| EQ-5D visual analog scale | 67.7 ± 16.6 | 70.3 ± 16.8 | 69.8 ± 17.2 |
Data are presented as median (IQR), mean ± standard deviation, or n (%).
Abbreviations: PCI, percutaneous coronary intervention; CABG, coronary artery bypass grafting; LVEF, left ventricular ejection fraction; CCTA, Cardiac Computed Tomographic Angiography; SAQ, Seattle Angina Questionnaire.
Defined as ≥3 of the following: prior myocardial infarction, prior revascularization, stroke, valvular heart disease, heart failure, atrial fibrillation, diabetes, smoking, hypertension, eGFR<60 (ml/min/m2), or chronic lung disease.
Participants undergoing coronary CT angiography for the study were required to have at least one ≥50% stenosis to qualify for randomization.
GDMT treatment goals at the end of follow-up are presented by age group in Table 2 and by treatment group in Supplemental Table S3. Adults ≥75 years old were less likely to achieve the composite 7-item GDMT goal compared with adults 65–74 years old and <65 years old. Adults <65 and 65–74 years old were more likely to attain the SBP goal of <140 mmHg and to be taking a high-intensity statin dose than adults ≥75 years old, but adults 65–74 and ≥75 years old were more likely to have had an LDL-C<70 mg/dL and quit smoking during the study. Rates of cardiac catheterization and revascularization overall and within 90 days of randomization by age group are presented in Table 2 and by treatment group in Supplemental Table S3. Of participants in the invasive group, 1034/1099 (94.1%) adults <65 years old, 822/858 (92.9%) adults 65–74 years old, and 325/338 (96.2%) adults ≥75 years old underwent cardiac catheterization; however, adults ≥75 years old (236/338, 69.8%) were less likely to be revascularized than adults <65 years old (919/1099, 83.6%) and adults 65–74 years old (695/858, 81.0%). Regarding reasons revascularization was not performed in the invasive group, absence of obstructive CAD was more common in younger adults, and coronary anatomy unsuitable for any mode of revascularization was more common in older adults (Supplemental Table S3). Of the participants in the conservative group who underwent revascularization, adults ≥75 years old (26/59, 44.1%) were more likely to do so against study protocol (e.g., revascularization in the absence of an MI or intolerable symptoms) than adults <65 years old (86/266, 32.3%) and 65–74 years old (75/198, 37.9%).
Table 2.
GDMT and invasive management at the end of follow-up by age group.
| Age <65 | Age 65–74 | Age ≥75 | |
|---|---|---|---|
| (N=2,239) | (N=1,713) | (N=665) | |
|
| |||
| Guideline Directed Medical Therapy | |||
| Not smoking | 1807/2128 (84.9%) | 1504/1621 (92.8%) | 584/599 (97.5%) |
| Systolic blood pressure <140 mmHg | 1735/2179 (79.6%) | 1262/1670 (75.6%) | 450/627 (71.8%) |
| LDL-C <70 mg/dL and taking statin | 1142/2129 (53.6%) | 1008/1651 (61.1%) | 376/614 (61.2%) |
| Aspirin or aspirin alternative | 2115/2190 (96.6%) | 1625/1678 (96.8%) | 616/633 (97.3%) |
| High-intensity statin | 1429/2190 (65.3%) | 1009/1678 (60.1%) | 330/633 (52.1%) |
| ACE inhibitor/ARB* | 1621/2046 (79.2%) | 1248/1589 (78.5%) | 462/612 (75.5%) |
| Beta blockers | 1597/1653 (96.6%) | 1197/1262 (94.8%) | 447/477 (93.7%) |
| Overall GDMT goal met | 344/1546 (22.3%) | 246/1196 (20.6%) | 80/434 (18.4%) |
| Invasive Treatment in Both Strategies | |||
| Catheterization | |||
| Any | 1350/2239 (60.3%) | 1064/1713 (62.1%) | 405/665 (60.9%) |
| Within 90 days of randomization | 1058/2239 (47.3%) | 848/1713 (49.5%) | 336/665 (50.5%) |
| Revascularization | |||
| Any | 1185/2239 (52.9%) | 893/1713 (52.1%) | 295/665 (44.4%) |
| Within 90 days of randomization | 877/2239 (39.2%) | 650/1713 (37.9%) | 217/665 (32.6%) |
| Coronary artery bypass graft surgery | |||
| Any | 312/2239 (13.9%) | 304/1713 (17.7%) | 89/665 (13.4%) |
| Within 90 days of randomization | 176/2239 (7.9%) | 170/1713 (9.9%) | 55/665 (8.3%) |
| Percutaneous coronary intervention | |||
| Any | 904/2239 (40.4%) | 648/1713 (37.8%) | 215/665 (32.3%) |
| Within 90 days of randomization | 709/2239 (31.7%) | 493/1713 (28.8%) | 163/665 (24.5%) |
Abbreviations: ACE, angiotensin-converting enzyme; ARB, angiotensin II receptor blocker; LDL-C, low-density lipoprotein cholesterol; GDMT, guideline-directed medical therapy
Includes class I and non-class I indications
Health Status Outcomes
Unadjusted SAQ Summary Scores, SAQ domains, RDS, and EQ-5D VAS scores by age group over time are presented in Supplemental Figure S1, Supplemental Figure S2, and Supplemental Table S4. Older patients had higher baseline SAQ Summary scores, SAQ Angina Frequency scores, and SAQ QOL scores, and were more likely to be angina-free. Conversely, older patients reported greater physical limitations due to angina than younger patients.
Crude health status outcomes with 95% CIs across the age spectrum by treatment group at 12-months are presented in Figure 1. There was greater separation of the curves, favoring an invasive strategy, for younger patients for SAQ Summary Score and SAQ Quality of Life. This trend was also observed for SAQ Physical Limitations and EQ-5D VAS scores; however, the magnitude of separation with younger age was smaller. For SAQ Angina Frequency, an invasive strategy was superior to conservative strategy across all ages.
Figure 1. Unadjusted 1-year health status outcomes by treatment group as a function of age.
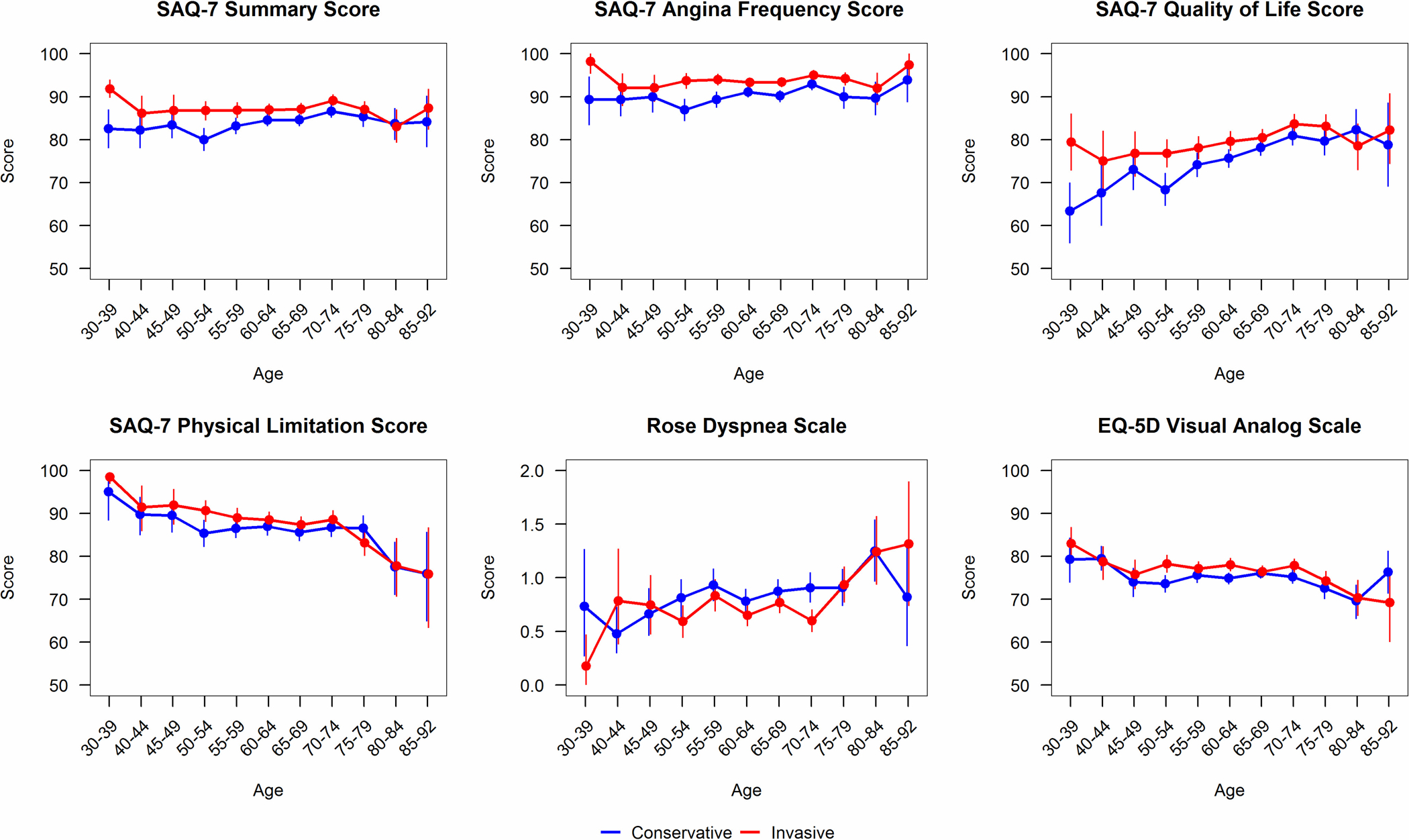
Health status with an invasive strategy is represented in red, and with a conservative strategy in blue. Vertical bars represent 95% confidence intervals. Abbreviations: SAQ, Seattle Angina Questionnaire. Higher scores better for SAQ, EQ-5D Visual Analog Scale; lower scores better for Rose Dyspnea Scale.
In the multivariable analyses, there were greater health status benefits with invasive management in younger participants versus older participants in the model without age-related comorbidities (Supplemental Figure S3) and the fully adjusted model including age-related comorbidities (Central Illustration). In the fully adjusted model, the age-by-treatment interaction was significant for the SAQ Summary Score (p for interaction = 0.008) and SAQ Quality of Life (p for interaction = 0.02), but not for SAQ Angina Frequency (p for interaction = 0.08), SAQ Physical Limitation (p for interaction = 0.15), RDS (p for interaction = 0.47), or EQ-5D VAS (p for interaction = 0.36) scores. The difference in the SAQ Summary Score (invasive minus conservative) was 4.90 (95% CI 3.56, 6.24) at age 55, 3.48 (95% CI 2.40, 4.57) at age 65, and 2.13 (95% CI 0.75, 3.51) at age 75. Point estimates for the other health status outcomes are presented in Supplemental Table S5 for both models. In the sensitivity analysis excluding 1640 patients without baseline angina, the health status models showed similar age-related trends, with greater benefits in younger participants undergoing an invasive strategy and less incremental benefit with invasive treatment with respect to SAQ Summary and SAQ QOL scores in older participants (Supplemental Figure S4 and S5).
Central Illustration. Interaction between age and health status treatment difference.
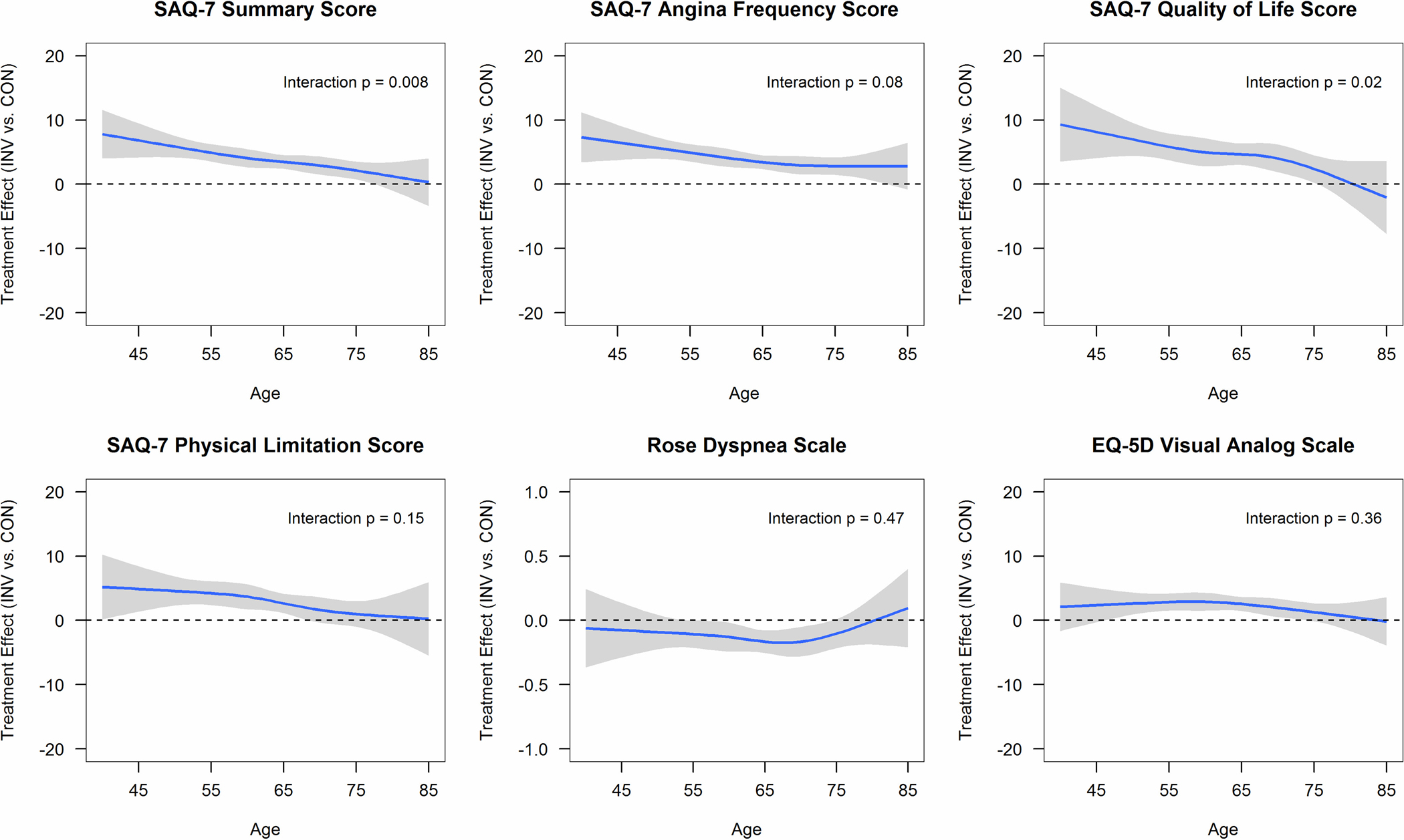
Association between age and health status treatment difference (invasive [INV] minus conservative [CON]) using multivariable linear regression and adjusting for geographic region, sex, smoking, body mass index, hypertension, degree of ischemia, number of diseased vessels (≥70%), congestive heart failure, New York Heart Association class, diabetes, estimated glomerular filtration rate, prior myocardial infarction, prior percutaneous coronary intervention, prior coronary artery bypass grafting, peripheral vascular disease, stroke/transient ischemic attack, and baseline health status. Positive values favor invasive treatment for SAQ, EQ-5D VAS; negative values favor invasive treatment for Rose Dyspnea Scale. Abbreviations: SAQ, Seattle Angina Questionnaire.
Clinical Events
Over a median follow-up of 3.2 years (IQR 2.2, 4.4), the cumulative event rate for the primary clinical outcome was 12.5% in participants <65 years old, 15.0% in participants 65–74 years old, and 21.1% in participants ≥75 years old. In unadjusted analyses, compared with participants <65 years old, participants 65–74 years old had a more than 20% increased risk (HR 1.24, 95% CI 1.04, 1.49) and participants ≥75 years old had an almost two-fold increased risk (HR 1.95, 95% CI 1.58–2.41) of experiencing the primary clinical outcome (p<0.001). The risk of CV death, non-procedural MI, hospitalization for heart failure, and stroke also increased with increasing age, but the risk of procedural MI did not (Supplemental Table S6).
Multivariable-adjusted Cox proportional hazards models for the primary clinical outcome showed no difference in cause-specific hazard ratios by management strategy across the age spectrum in the model without age-related comorbidities (Supplemental Figure S6) and the fully adjusted model (Figure 2, Panel A). In the fully adjusted model, the cause-specific HR for the primary clinical outcome at age 55 was 0.81 (95% CI 0.62 to 1.07), 0.90 (95% CI 0.76, 1.06) at age 65, and 0.99 (95% CI 0.80, 1.22) at age 75, with an interaction p-value of 0.29. All age-by-treatment interaction p-values for the other clinical events were non-significant (Figure 2, Panels A and B; Supplemental Figures S6 and S7). Invasive management was associated with a decrease in non-procedural MI but an increase in procedural MI that was consistent across the age spectrum (Figure 2, Panel C; Supplemental Figure S8).
Figure 2. Association between treatment strategy and clinical events by age group.
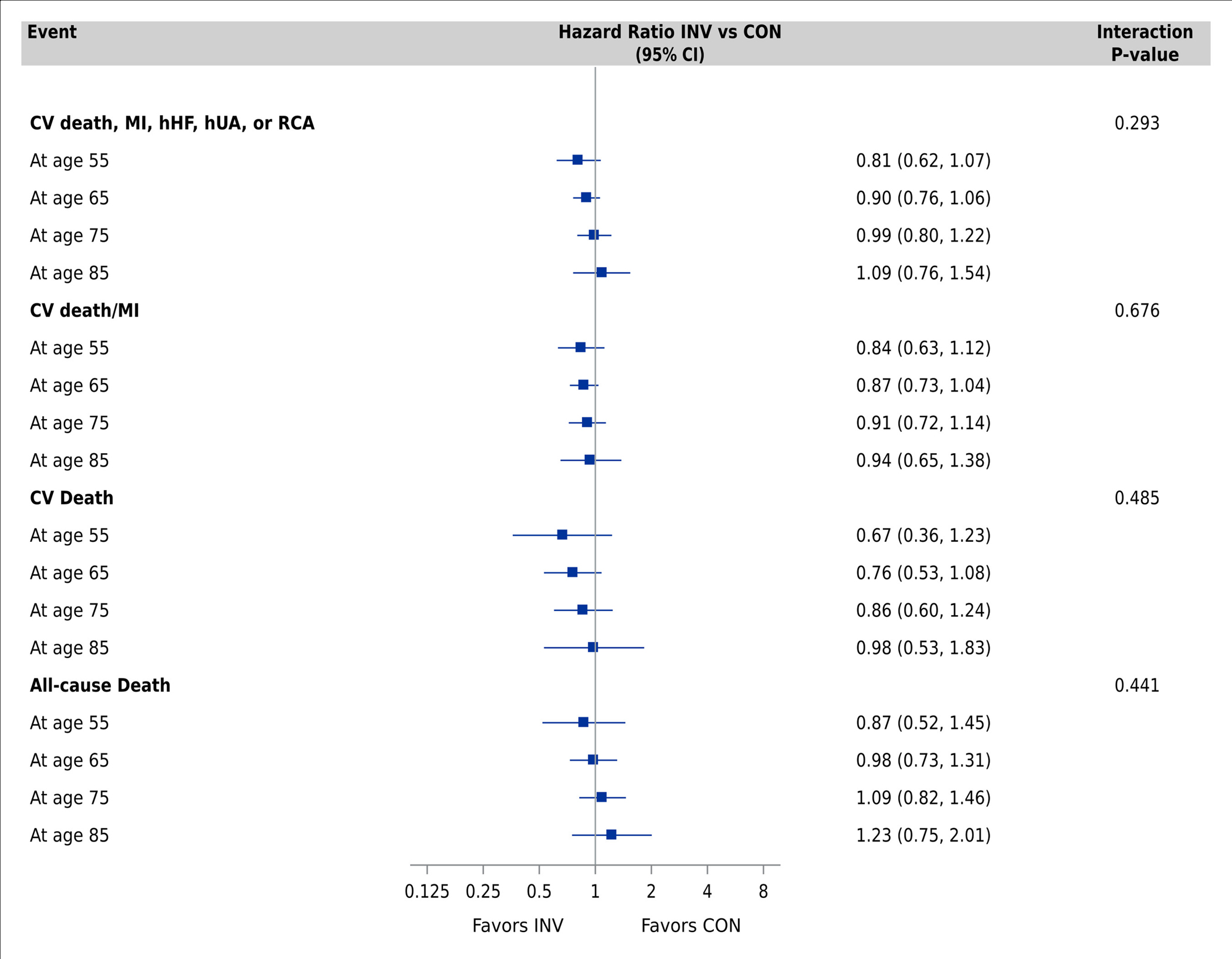
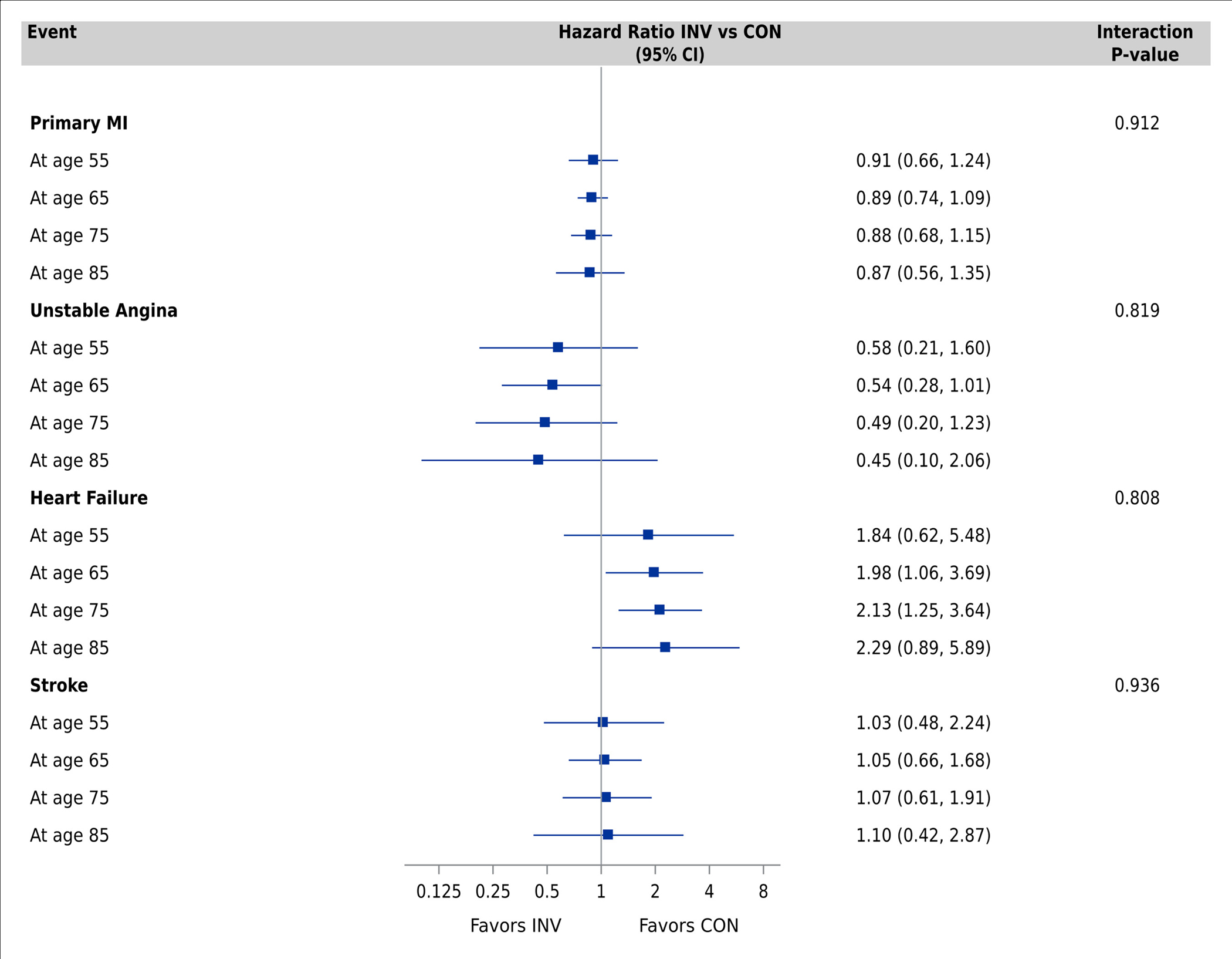
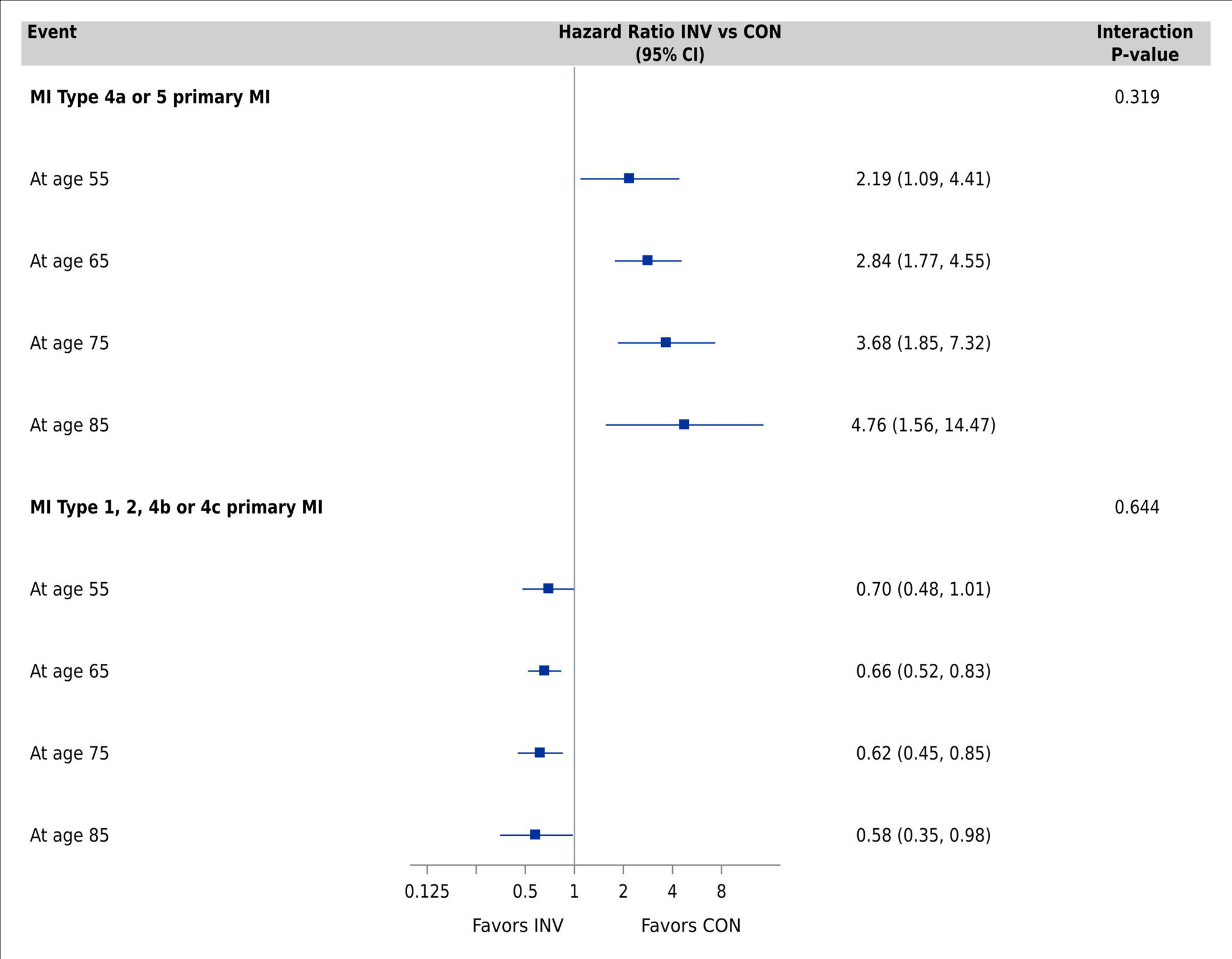
Comparisons performed using Cox proportional hazards models adjusting for geographic region, sex, smoking, body mass index, hypertension, degree of ischemia, number of diseased vessels (≥70%), congestive heart failure, New York Heart Association class, diabetes, estimated glomerular filtration rate, prior myocardial infarction, prior percutaneous coronary intervention, prior coronary artery bypass grafting, peripheral vascular disease, and stroke/transient ischemic attack. Panel A: Primary clinical outcome, CV death/MI, CV death, and all-cause death. Panel B: MI, hospitalization for unstable angina and heart failure, stroke. Panel C: Procedural MI (MI type 4a or 5) and non-procedural MI (MI type 1, 2, 4b or 4c) Abbreviations: CI, confidence interval; CV, cardiovascular; MI, myocardial infarction; hHF, hospitalization for heart failure; hUA, hospitalization for unstable angina; RCA, resuscitated cardiac arrest; INV, invasive; CON, conservative.
DISCUSSION
Although older adults with CCD experience higher mortality and complication rates with revascularization than younger adults3–6, the decision to pursue revascularization may be particularly compelling if it improves angina-related quality of life. To better inform shared decision-making for proceeding to angiography and potential revascularization in older adults with CCD, we examined the association of an invasive treatment strategy with health status and clinical outcomes of older adults in this ISCHEMIA sub-group analysis. We found that an invasive strategy was associated with smaller gains in angina-related health status and quality of life in older patients as compared with younger patients, although there were no differences in the magnitude of benefit across age groups in terms of angina frequency and physical functioning. Older age was associated with an increased risk of CV death, non-procedural MI, hospitalization for heart failure, and stroke, but not procedural MI. Consistent with the main trial results, we found that an invasive treatment strategy did not reduce event rates in either older or younger adults compared with conservative treatment. Our findings provide new insights into how best to describe the benefits of invasive treatment strategies as a function of age and underscore the importance of shared decision-making to elicit patients’ treatment preferences, particularly in older patients.
Prior Studies
Several major clinical trials in the last 20 years have examined the treatment benefits of an invasive versus conservative strategy in older adults. The Trial of Invasive Versus Medical Therapy in Elderly Patients with Chronic Symptomatic Coronary Artery Disease (TIME) exclusively enrolled 305 adults ≥75 years old and found that revascularization resulted in rapid improvements in angina severity and QOL measures compared with GDMT, but was associated with an early increased hazard for death or MI over 12-months of follow-up.7,28 At long-term follow-up (median 4.1 years), there was no difference in mortality rates, MI rates, or QOL.29 A prespecified age-stratified analysis of the COURAGE trial (Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation), which defined older adults as ≥65 years old (n=904; 40% of trial population), found no interaction between age and treatment effect with respect to angina-free status or clinical events at 5 years.30 The BARI 2D (Bypass Angioplasty Revascularization Investigation 2 Diabetes) trial reported no differences in all-cause mortality or cardiovascular events with revascularization (PCI or CABG) in participants with coronary artery disease and type 2 diabetes,9 and no interaction by age (≥70 years old; n=514, 22%) in terms of angina-free status, CV death, or MI at three years.31 Notably, our analysis included a substantially larger number of older adults than any of these prior trials; therefore, it had greater power to detect heterogeneity of treatment benefit by age. Our findings of no age interaction with treatment strategy regarding angina relief and clinical events aligns well with these previous studies and extends the evidence to patients with moderate or severe ischemia. However, while there were no differences in angina or clinical events, we found less benefits in older participants with respect to angina-related health status and quality of life.
Our findings are congruent with previous reports describing a relatively preserved QOL in older adults, despite significant symptom burden and physical limitations.32,33 For example, older adults with heart failure have fairly preserved QOL, despite more functional limitations than younger adults.34 Yet, this study also highlighted that decline in physical functioning was more detrimental to older patients’ perceived QOL, underscoring the importance of serially monitoring the health status of older adults with cardiovascular disease. The degree to which angina relief affects physical functioning and QOL in older adults with CCD compared with younger adults has not been previously studied but warrants further investigation.
Clinical interpretation
The health status findings of this analysis may be best understood through the framework by which the SAQ was developed.21 In a typical patient with CCD, myocardial ischemia results in anginal symptoms (SAQ Angina Frequency scale) that can impair a patient’s physical functioning (SAQ Physical Limitation scale), which limits how they desire to be feeling and decreases their QOL (SAQ Quality of Life scale). We posit that while the benefits of an invasive strategy in reducing angina and improving physical function were similar in older and younger patients, the importance of these benefits may be less critical to older patients who may have fewer physical demands and are more readily able to adapt to angina than younger patients, thus resulting in less difference in quality of life between the two treatment strategies in older patients.
As revascularization has become increasingly safe, periprocedural PCI and CABG mortality rates have declined across all age groups35–37, with the largest reduction observed in patients ≥70 years old.35 Yet, older age increases the risk of periprocedural bleeding5 and acute kidney injury4, discussions that should be included in the shared decision-making process. Moreover, our findings demonstrate that angina relief should be considered along with other health status outcomes, including physical functioning and QOL, particularly in older adults. Careful consideration (and if possible, quantification with the SAQ) of anginal symptoms, physical functioning, and QOL could be used to align treatment decisions with care goals. To meet this goal, research examining the heterogeneity of health status treatment benefits from ISCHEMIA to develop a shared decision-making tool is ongoing. Moreover, geriatric-centric factors such as cognitive function38,39, frailty40,41, and independence42 can influence clinical and health status outcomes and should be considered as additional factors for such risk models and shared decision-making tools.
Limitations
Our analysis has some limitations. First, older adults in the ISCHEMIA trial may not be reflective of older adults encountered in clinical practice.43,44 For example, patients with LVEF ≤35%, eGFR <30 mL/min/1.73 m2, or unprotected left main disease ≥50%, conditions that are more prevalent in older adults, were excluded from the ISCHEMIA trial. Clinical trial participants may be more likely to support research and be connected to the health system, resulting in them being distinct from among those with similar characteristics in the general population. Moreover, our findings do not extend to individuals with severe angina or recent ACS who were also excluded from the ISCHEMIA trial. Second, geriatric-related conditions (e.g., frailty, cognitive status, and functional independence) were not assessed in the ISCHEMIA trial; thus, they were not included in our adjusted models, although these conditions should be balanced between treatment groups due to randomization. Furthermore, these conditions are associated with increased mortality and delayed recovery from revascularization, making a conservative strategy potentially even more attractive for older frail adults. Third, there were disproportionally more White participants in the older cohorts, potentially limiting the generalizability to older Black, Hispanic, or Asian patients. Fourth, although ISCHEMIA enrolled more participants ≥75 years old than previous strategy trials for CCD, there were relatively fewer participants at the extremes of age, leading to wider confidence intervals and more uncertainty in the clinical and health status outcome point estimates among the youngest and oldest patients.
CONCLUSION
Among participants with CCD and moderate or severe ischemia, we found that the health status benefits with an invasive strategy differed across age groups in magnitude of benefit. Although invasive treatment resulted in larger and more rapid improvements in angina in all age groups, older participants experienced smaller improvements in angina-related health status and QOL with an invasive approach compared with younger participants. There were no differences by age in clinical event rates with an invasive versus conservative treatment strategy. This knowledge can help guide shared decision-making discussions between clinicians and older adults with CCD.
PERSPECTIVES
Competency in Patient Care and Procedural Skills:
Older adults with chronic coronary disease may gain less improvement in health status and quality of life than younger patients from addition of revascularization to guideline-directed medical therapy.
Translational outlook:
Further research is needed to examine the interdependence of frailty, cognitive function, and functional independence with clinical outcomes after revascularization in older adults with chronic coronary disease.
Supplementary Material
SOURCES OF FUNDING
This project was supported in part by NIH grants U01HL105907, U01HL105462, U01HL105561, and U01HL105565; and in part by Clinical Translational Science Award numbers 11UL1 TR001445 and UL1 TR002243 from the National Center for Advancing Translational Sciences. Dr. Nguyen is currently supported by the National Heart, Lung, and Blood Institute under Award Number T32H110837. The contents of this project are solely the responsibility of the authors and do not necessarily represent official views of the National Center for Advancing Translational Sciences; the National Heart, Lung, and Blood Institute; the National Institutes of Health; or the Department of Health and Human Services.
Abbreviations:
- ISCHEMIA
International Study of Comparative Health Effectiveness with Medical and Invasive Approaches
- CCD
Chronic coronary disease
- PCI
Percutaneous coronary intervention
- CABG
Coronary artery bypass grafting
- GDMT
Guideline-directed medical therapy
- CCTA
Coronary computed tomography angiography
- SAQ
Seattle Angina Questionnaire
- QOL
Quality of life
- RDS
Rose Dyspnea Scale
Footnotes
DISCLOSURES
Dr. Spertus reports grants from National Heart, Lung, and Blood Institute during the conduct of the study; personal fees from Bayer, personal fees from Novartis, personal fees from AstraZeneca, personal fees from Amgen, personal fees from Janssen, personal fees from United Healthcare, grants from American College of Cardiology, outside the submitted work; In addition, Dr. Spertus has a patent Copyright to Seattle Angina Questionnaire with royalties paid and Board of Directors for Blue Cross Blue Shield of Kansas City and Equity in Health Outcomes Sciences. Dr. Newman reports grants from National Heart, Lung, and Blood Institute during the conduct of the study. Dr. Alexander reports grants from National Heart, Lung, and Blood Institute during the conduct of the study. Mr. Jones reports employment by the National Heart, Lung, and Blood Institute during the conduct of the study. Mrs. Stevens reports grants from National Heart, Lung, and Blood Institute during the conduct of the study. Dr. O’Brien reports grants from National Heart, Lung, and Blood Institute during the conduct of the study. Dr. Gamma reports grants from National Heart, Lung, and Blood Institute during the conduct of the study. Dr. Perna reports grants from National Heart, Lung, and Blood Institute during the conduct of the study. Dr. Garg reports grants from National Heart, Lung, and Blood Institute during the conduct of the study. Dr. Chow holds the Saul and Edna Goldfarb Chair in Cardiac Imaging Research. He receives research support from TD Bank, CV Diagnostix and AusculSciences, and Siemens Healthineers. He has equity interest in General Electric. Dr. Vertes reports grants from National Heart, Lung, and Blood Institute during the conduct of the study. Dr. White reports grants from National Heart, Lung, and Blood Institute during the conduct of the study; reports receiving grant support paid to the institution and fees for serving on a steering committee for the ODYSSEY OUTCOMES trial (Evaluation of Cardiovascular Outcomes After an Acute Coronary Syndrome During Treatment With Alirocumab) from Sanofi-Aventis and Regeneron Pharmaceuticals, for the ACCELERATE study (A Study of Evacetrapib in High-Risk Vascular Disease) from Eli Lilly, for the STRENGTH trial (Outcomes Study to Assess Statin Residual Risk Reduction With EpaNova in High CV Risk Patients With Hypertriglyceridemia) from Omthera Pharmaceuticals, for the HEART-FID study (Randomized Placebo-Controlled Trial of FCM as Treatment for Heart Failure With Iron Deficiency) from American Regent; for the CAMELLIA-TIMI study (A Study to Evaluate the Effect of Long-term Treatment With BELVIQ [Lorcaserin HC] on the Incidence of Major Adverse Cardiovascular Events and Conversion to Type 2 Diabetes Mellitus in Obese and Overweight Subjects With Cardiovascular Disease or Multiple Cardiovascular Risk Factors) from Eisai Inc, for the dal-GenE study (Effect of Dalcetrapib vs Placebo on CV Risk in a Genetically Defined Population With a Recent ACS) from DalCor Pharma UK Inc, for the AEGIS-II study from CSL Behring, for the SCORED trial (Effect of Sotagliflozin on Cardiovascular and Renal Events in Patients With Type 2 Diabetes and Moderate Renal Impairment Who Are at Cardiovascular Risk) and the SOLOIST-WHF trial (Effect of Sotagliflozin on Cardiovascular Events in Patients With Type 2 Diabetes Post Worsening Heart Failure) from Sanofi-Aventis Australia Pty Ltd, and for the CLEAR Outcomes Study (Evaluation of Major Cardiovascular Events in Patients With, or at High Risk for, Cardiovascular Disease Who Are Statin Intolerant Treated With Bempedoic Acid [ETC-1002] or Placebo) from Esperion Therapeutics Inc. He was on the Advisory Board for Genentech, Inc. and received lecture fees from AstraZeneca. Dr. Smanio reports grants from National Heart, Lung and Blood Institute during the conduct of the study. Dr. Senior reports grants from National Heart, Lung and Blood Institute during the conduct of the study. Dr. Held reports grants from National Heart, Lung, and Blood Institute during the conduct of the study. Dr. William E. Boden reports grants from National Heart, Lung, and Blood Institute during the conduct of the study; grants from Abbvie, grants from Amarin, grants from Amgen, personal fees from Amgen, personal fees from Cleveland Clinic Clinical Coordinating Center, and personal fees from Janssen, outside the submitted work. Dr. Mark reports grants from National Heart, Lung, and Blood Institute during the conduct of the study; grants from HeartFlow, and grants from Merck, outside the submitted work. Dr. Reynolds received funding for this research from the National Heart, Lung, and Blood Institute, and received in-kind donations for unrelated research from Abbott Vascular, Siemens and Philips. Dr. Bangalore reports grants from the National Heart, Lung, and Blood Institute and Abbott Vascular, and serves on the advisory board for Abbott Vascular, Pfizer, Amgen, Biotronik, Meril and Reata. Dr. Chan receives funding from the American Heart Association and is a consultant for Optum Rx. Dr. Stone reports grants and personal fees from the National Heart, Lung, and Blood Institute during the conduct of the study; personal fees from Terumo, Amaranth, and Shockwave; personal fees and other from Valfix; personal fees from TherOx, Reva, Vascular Dynamics, Robocath, HeartFlow, Gore, Ablative Solutions, Matrizyme, Miracor, Neovasc, V-wave, Abiomed, Claret, and Sirtex; personal fees and other from Ancora and Qool Therapeutics; other from Cagent, Applied Therapeutics, Biostar family of funds, and MedFocus family of funds; personal fees and other from SpectraWave; personal fees from MAIA Pharmaceuticals; personal fees and other from Orchestra Biomed; other from Aria; personal fees from Vectorious; and other from Cardiac Success, outside the submitted work. Dr. Maron reports grants from National Heart, Lung, and Blood Institute during the conduct of the study. Dr. Hochman is PI for the ISCHEMIA trial for which, in addition to support by National Heart, Lung, and Blood Institute grant, devices and medications were provided by Abbott Vascular; Medtronic, Inc.; Abbott Laboratories (formerly St. Jude Medical, Inc); Royal Philips NV (formerly Volcano Corporation); Arbor Pharmaceuticals, LLC; AstraZeneca Pharmaceuticals, LP; Merck Sharp & Dohme Corp.; Omron Healthcare, Inc, Sunovion Pharmaceuticals, Inc. Espero BioPharma; and Amgen, Inc; and financial donations from Arbor Pharmaceuticals LLC and AstraZeneca Pharmaceuticals LP. The remaining authors have nothing to disclose.
Trial Registration: NCT01471522; https://clinicaltrials.gov
REFERENCES
- 1.Fihn SD, Gardin JM, Abrams J, et al. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS Guideline for the Diagnosis and Management of Patients With Stable Ischemic Heart Disease. Circulation. 2012;126(25):e354–e471. doi: 10.1161/CIR.0b013e318277d6a0 [DOI] [PubMed] [Google Scholar]
- 2.Tegn N, Eek C, Abdelnoor M, et al. Patients aged 80 years or older with non-ST-elevation myocardial infarction or unstable angina pectoris randomised to an invasive versus conservative strategy: angiographic and procedural results from the After Eighty study. Open Hear. 2020;7(2):e001256. doi: 10.1136/openhrt-2020-001256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peterson ED, Dai D, DeLong ER, et al. Contemporary mortality risk prediction for percutaneous coronary intervention: results from 588,398 procedures in the National Cardiovascular Data Registry. J Am Coll Cardiol. 2010;55(18):1923–1932. doi: 10.1016/j.jacc.2010.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tsai TT, Patel UD, Chang TI, et al. Validated contemporary risk model of acute kidney injury in patients undergoing percutaneous coronary interventions: insights from the National Cardiovascular Data Registry Cath-PCI Registry. J Am Heart Assoc. 2014;3(6):e001380. doi: 10.1161/JAHA.114.001380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rao SV, McCoy LA, Spertus JA, et al. An updated bleeding model to predict the risk of post-procedure bleeding among patients undergoing percutaneous coronary intervention: a report using an expanded bleeding definition from the National Cardiovascular Data Registry CathPCI Registry. JACC Cardiovasc Interv. 2013;6(9):897–904. doi: 10.1016/j.jcin.2013.04.016 [DOI] [PubMed] [Google Scholar]
- 6.Shahian DM, O’Brien SM, Filardo G, et al. The Society of Thoracic Surgeons 2008 cardiac surgery risk models: part 1--coronary artery bypass grafting surgery. Ann Thorac Surg. 2009;88(1 Suppl):S2–22. doi: 10.1016/j.athoracsur.2009.05.053 [DOI] [PubMed] [Google Scholar]
- 7.Pfisterer M, Buser P, Osswald S, et al. Outcome of elderly patients with chronic symptomatic coronary artery disease with an invasive vs optimized medical treatment strategy: one-year results of the randomized TIME trial. JAMA. 2003;289(9):1117–1123. doi: 10.1001/jama.289.9.1117 [DOI] [PubMed] [Google Scholar]
- 8.Boden WE, O’Rourke RA, Teo KK, et al. Optimal Medical Therapy with or without PCI for Stable Coronary Disease. N Engl J Med. 2007;356(15):1503–1516. doi: 10.1056/NEJMoa070829 [DOI] [PubMed] [Google Scholar]
- 9.A Randomized Trial of Therapies for Type 2 Diabetes and Coronary Artery Disease. N Engl J Med. 2009;360(24):2503–2515. doi: 10.1056/NEJMoa0805796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nanna MG, Peterson ED, Wu A, et al. Age, knowledge, preferences, and risk tolerance for invasive cardiac care. Am Heart J. 2020;219:99–108. doi: 10.1016/j.ahj.2019.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tsevat J, Dawson NV, Wu AW, et al. Health values of hospitalized patients 80 years or older. HELP Investigators. Hospitalized Elderly Longitudinal Project. JAMA. 1998;279(5):371–375. doi: 10.1001/jama.279.5.371 [DOI] [PubMed] [Google Scholar]
- 12.Maron DJ, Hochman JS, Reynolds HR, et al. Initial Invasive or Conservative Strategy for Stable Coronary Disease. N Engl J Med. 2020;382(15):1395–1407. doi: 10.1056/NEJMoa1915922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spertus JA, Jones PG, Maron DJ, et al. Health-Status Outcomes with Invasive or Conservative Care in Coronary Disease. N Engl J Med. 2020;382(15):1408–1419. doi: 10.1056/NEJMoa1916370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Organization WH. World Report on Ageing and Health. Geneva PP - Geneva: World Health Organization; https://apps.who.int/iris/handle/10665/186463. [Google Scholar]
- 15.Maron DJ, Hochman JS, O’Brien SM, et al. International Study of Comparative Health Effectiveness with Medical and Invasive Approaches (ISCHEMIA) trial: Rationale and design. Am Heart J. 2018;201:124–135. doi: 10.1016/j.ahj.2018.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chan PS, Jones PG, Arnold SA, Spertus JA. Development and Validation of a Short Version of the Seattle Angina Questionnaire. Circ Cardiovasc Qual Outcomes. 2014;7(5):640–647. doi: 10.1161/CIRCOUTCOMES.114.000967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Spertus JA, Winder JA, Dewhurst TA, Deyo RA, Fihn SD. Monitoring the quality of life in patients with coronary artery disease. Am J Cardiol. 1994;74(12):1240–1244. doi: 10.1016/0002-9149(94)90555-X [DOI] [PubMed] [Google Scholar]
- 18.Spertus JA, Winder JA, Dewhurst TA, et al. Development and evaluation of the Seattle Angina questionnaire: A new functional status measure for coronary artery disease. J Am Coll Cardiol. 1995;25(2):333–341. doi: 10.1016/0735-1097(94)00397-9 [DOI] [PubMed] [Google Scholar]
- 19.Spertus JA, Jones P, McDonell M, Fan V, Fihn SD. Health Status Predicts Long-Term Outcome in Outpatients With Coronary Disease. Circulation. 2002;106(1):43–49. doi: 10.1161/01.CIR.0000020688.24874.90 [DOI] [PubMed] [Google Scholar]
- 20.Arnold SV, Morrow DA, Lei Y, et al. Economic Impact of Angina After an Acute Coronary Syndrome. Circ Cardiovasc Qual Outcomes. 2009;2(4):344–353. doi: 10.1161/CIRCOUTCOMES.108.829523 [DOI] [PubMed] [Google Scholar]
- 21.Thomas M, Jones PG, Arnold SV, Spertus JA. Interpretation of the Seattle Angina Questionnaire as an Outcome Measure in Clinical Trials and Clinical Care: A Review. JAMA Cardiol. 2021;6(5):593–599. doi: 10.1001/jamacardio.2020.7478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arnold SV, Kosiborod M, Li Y, et al. Comparison of the Seattle Angina Questionnaire With Daily Angina Diary in the TERISA Clinical Trial. Circ Cardiovasc Qual Outcomes. 2014;7(6):844–850. doi: 10.1161/CIRCOUTCOMES.113.000752 [DOI] [PubMed] [Google Scholar]
- 23.EuroQol--a new facility for the measurement of health-related quality of life. Health Policy. 1990;16(3):199–208. doi: 10.1016/0168-8510(90)90421-9 [DOI] [PubMed] [Google Scholar]
- 24.Rose GA, Blackburn H. Cardiovascular survey methods. Monogr Ser World Health Organ. 1968;56:1–188. [PubMed] [Google Scholar]
- 25.Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD. Third Universal Definition of Myocardial Infarction. Circulation. 2012;126(16):2020–2035. doi: 10.1161/CIR.0b013e31826e1058 [DOI] [PubMed] [Google Scholar]
- 26.Buuren S, Groothuis-Oudshoorn C. MICE: Multivariate Imputation by Chained Equations in R. J Stat Softw. 2011;45. doi: 10.18637/jss.v045.i03 [DOI] [Google Scholar]
- 27.Rubin DB. Multiple Imputation for Nonresponse in Surveys. New York: John Wiley and Sons [Google Scholar]
- 28.Pfisterer M, Buser P, Osswald S, et al. Outcome of Elderly Patients With Chronic Symptomatic Coronary Artery Disease With an Invasive vs Optimized Medical Treatment StrategyOne-Year Results of the Randomized TIME Trial. JAMA. 2003;289(9):1117–1123. doi: 10.1001/jama.289.9.1117 [DOI] [PubMed] [Google Scholar]
- 29.Pfisterer M Long-Term Outcome in Elderly Patients With Chronic Angina Managed Invasively Versus by Optimized Medical Therapy. Circulation. 2004;110(10):1213–1218. doi: 10.1161/01.CIR.0000140983.69571.BA [DOI] [PubMed] [Google Scholar]
- 30.Teo KK, Sedlis SP, Boden WE, et al. Optimal Medical Therapy With or Without Percutaneous Coronary Intervention in Older Patients With Stable Coronary Disease. A Pre-Specified Subset Analysis of the COURAGE (Clinical Outcomes Utilizing Revascularization and Aggressive druG Evaluation) Trial. J Am Coll Cardiol. 2009;54(14):1303–1308. doi: 10.1016/j.jacc.2009.07.013 [DOI] [PubMed] [Google Scholar]
- 31.Chung S-C, Hlatky MA, Faxon D, et al. The effect of age on clinical outcomes and health status BARI 2D (Bypass Angioplasty Revascularization Investigation in Type 2 Diabetes). J Am Coll Cardiol. 2011;58(8):810–819. doi: 10.1016/j.jacc.2011.05.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cockerham WC, Sharp K, Wilcox JA. Aging and perceived health status. J Gerontol. 1983;38(3):349–355. doi: 10.1093/geronj/38.3.349 [DOI] [PubMed] [Google Scholar]
- 33.Johnson RJ, Wolinsky FD. The Structure of Health Status Among Older Adults: Disease, Disability, Functional Limitation, and Perceived Health. J Health Soc Behav. 1993;34(2):105–121. doi: 10.2307/2137238 [DOI] [PubMed] [Google Scholar]
- 34.Masoudi FA, Rumsfeld JS, Havranek EP, et al. Age, functional capacity, and health-related quality of life in patients with heart failure. J Card Fail. 2004;10(5):368–373. doi: 10.1016/j.cardfail.2004.01.009 [DOI] [PubMed] [Google Scholar]
- 35.Singh M, Peterson ED, Roe MT, et al. Trends in the Association Between Age and In-Hospital Mortality After Percutaneous Coronary Intervention. Circ Cardiovasc Interv. 2009;2(1):20–26. doi: 10.1161/CIRCINTERVENTIONS.108.826172 [DOI] [PubMed] [Google Scholar]
- 36.Elbadawi A, Elgendy IY, Ha LD, et al. National Trends and Outcomes of Percutaneous Coronary Intervention in Patients ≥70 Years of Age With Acute Coronary Syndrome (from the National Inpatient Sample Database). Am J Cardiol. 2019;123(1):25–32. doi: 10.1016/j.amjcard.2018.09.030 [DOI] [PubMed] [Google Scholar]
- 37.Ivanov J, Weisel RD, David TE, Naylor CD. Fifteen-Year Trends in Risk Severity and Operative Mortality in Elderly Patients Undergoing Coronary Artery Bypass Graft Surgery. Circulation. 1998;97(7):673–680. doi: 10.1161/01.CIR.97.7.673 [DOI] [PubMed] [Google Scholar]
- 38.Alexander KP, Newby LK, Cannon CP, et al. Acute Coronary Care in the Elderly, Part I. Circulation. 2007;115(19):2549–2569. doi: 10.1161/CIRCULATIONAHA.107.182615 [DOI] [PubMed] [Google Scholar]
- 39.Alexander KP, Newby LK, Armstrong PW, et al. Acute Coronary Care in the Elderly, Part II. Circulation. 2007;115(19):2570–2589. doi: 10.1161/CIRCULATIONAHA.107.182616 [DOI] [PubMed] [Google Scholar]
- 40.Singh M, Stewart R, White H. Importance of frailty in patients with cardiovascular disease. Eur Heart J. 2014;35(26):1726–1731. doi: 10.1093/eurheartj/ehu197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Afilalo J, Alexander KP, Mack MJ, et al. Frailty Assessment in the Cardiovascular Care of Older Adults. J Am Coll Cardiol. 2014;63(8):747–762. doi: 10.1016/j.jacc.2013.09.070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dodson JA, Arnold SV, Reid KJ, et al. Physical function and independence 1 year after myocardial infarction: observations from the Translational Research Investigating Underlying disparities in recovery from acute Myocardial infarction: Patients’ Health status registry. Am Heart J. 2012;163(5):790–796. doi: 10.1016/j.ahj.2012.02.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chatterjee S, Fanaroff AC, Parzynski C, et al. Comparison of Patients Undergoing Percutaneous Coronary Intervention in Contemporary U.S. Practice With ISCHEMIA Trial Population. Cardiovasc Interv. 2021;14(21):2344–2349. doi: 10.1016/J.JCIN.2021.08.047 [DOI] [PubMed] [Google Scholar]
- 44.De Luca L, Uguccioni M, Meessen J, et al. External applicability of the ISCHEMIA trial: An analysis of a prospective, nationwide registry of patients with stable coronary artery disease. EuroIntervention. 2020;16(12):E966–E973. doi: 10.4244/EIJ-D-20-00610 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


