Abstract
Background
The risk of incident atrial fibrillation (AF) among breast cancer survivors, especially for younger women, and cancer treatment effects on the association remain unclear. This study aimed to investigate the risk of AF among breast cancer survivors and evaluate the association by age group, length of follow-up, and cancer treatment.
Methods
Using data from the Korean Health Insurance Service database (2010–2017), 113,232 women newly diagnosed with breast cancer (aged ≥ 18 years) without prior AF history who underwent breast cancer surgery were individually matched 1:5 by birth year to a sample female population without cancer (n = 566,160) (mean[SD] follow-up, 5.1[2.1] years). Sub-distribution hazard ratios (sHRs) and 95% confidence intervals (CIs) considering death as a competing risk were estimated, adjusting for sociodemographic factors and cardiovascular/non-cardiovascular comorbidities.
Results
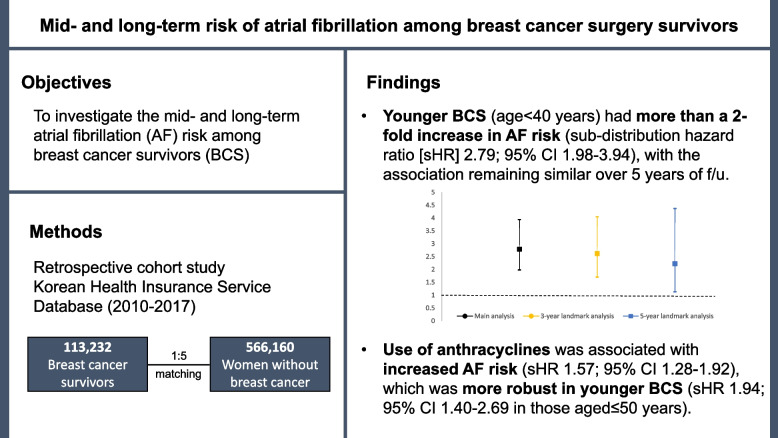
BCS had a slightly increased AF risk compared to their cancer-free counterparts (sHR 1.06; 95% CI 1.00–1.13), but the association disappeared over time. Younger BCS (age < 40 years) had more than a 2-fold increase in AF risk (sHR 2.79; 95% CI 1.98–3.94), with the association remaining similar over 5 years of follow-up. The increased risk was not observed among older BCS, especially those aged > 65 years. Use of anthracyclines was associated with increased AF risk among BCS (sHR 1.57; 95% CI 1.28–1.92), which was more robust in younger BCS (sHR 1.94; 95% CI 1.40–2.69 in those aged ≤ 50 years).
Conclusions
Our findings suggest that younger BCS had an elevated risk of incident AF, regardless of the length of follow-up. Use of anthracyclines may be associated with increased mid-to-long-term AF risk among BCS.
Graphical Abstract
Supplementary Information
The online version contains supplementary material available at 10.1186/s12916-024-03308-z.
Keywords: Atrial fibrillation, Breast cancer, Anthracyclines, Younger breast cancer survivors
Background
Individuals treated for breast cancer may be at increased risk of developing atrial fibrillation (AF), which is one of the key drivers of cardiovascular (CV) complications such as myocardial ischemia, stroke, and heart failure [1, 2]. Proposed mechanisms for increased risk of AF among breast cancer survivors include shared common risk factors such as advancing age, alcohol consumption, smoking, and obesity, as well as cancer-related proinflammatory state and cardiotoxic cancer treatment [1–3]. Due to increased early detection and improved survival following breast cancer treatment [4], mid- to long-term CV complications have become a major concern of breast cancer survivors [2].
The evidence regarding the risk of AF among breast cancer survivors has been primarily derived from studies limited by relatively small numbers of patients evaluated over a short period [5]. Additionally, most studies have focused on women over 65 years [6, 7] or have been limited by the short-term follow-up (e.g., < 3 years) [6, 8], small numbers of incident AF events [9], use of prevalent breast cancer cases [8, 10], early-stage breast cancer [11], and failure to consider competing risks (e.g., death) [8, 12, 13], and comprehensive cancer treatment effects [12–14]. Consequently, the risk of AF among breast cancer survivors is unknown.
Breast cancer and CV events in women vary with age, particularly before and after menopause onset [2]. For instance, compared to those without cancer, women aged < 40 years with breast cancer are at more than a 3-fold increased risk of developing CV complications [15], and women diagnosed with breast cancer before age 50 years are at a higher risk of developing treatment-related CV complications [16]. However, few studies have investigated whether AF risk differs between younger and older breast cancer survivors [8], particularly in Asian women, whose breast cancer incidence peaks in the mid to late 40 s, which is remarkably different from that observed in Western countries [17]. Using the Korean National Health Insurance Service (NHIS) database, we evaluated incident AF among breast cancer survivors compared to women without cancer, particularly investigating the mid- to long-term risk of AF and the role of cancer treatment on this association by the age at the time of breast cancer diagnosis.
Methods
Data source and study setting
The NHIS is a public health insurance program providing mandatory universal coverage to 97% of the South Korean population. The NHIS database comprises sociodemographic and claims-based healthcare information such as medical procedures, diagnoses, prescriptions, outpatient visits, and hospital admission [18, 19]. The NHIS also operates biennial standardized health screening examinations [20], which include past medical history, lifestyle behaviors (smoking, drinking, and physical activity), anthropometric measurements, and laboratory tests for nonemployees aged ≥ 40 years and employees regardless of age. The NHIS database has been validated for epidemiological and clinical research [21, 22].
This study was approved by the Samsung Medical Center (Seoul, South Korea; SMC 2020-03-108) Institutional Review Board. All information used for analyses was anonymized and de-identified; therefore, informed consent was not required. The database is open to all researchers whose study protocols are approved by the official review committee.
Study population
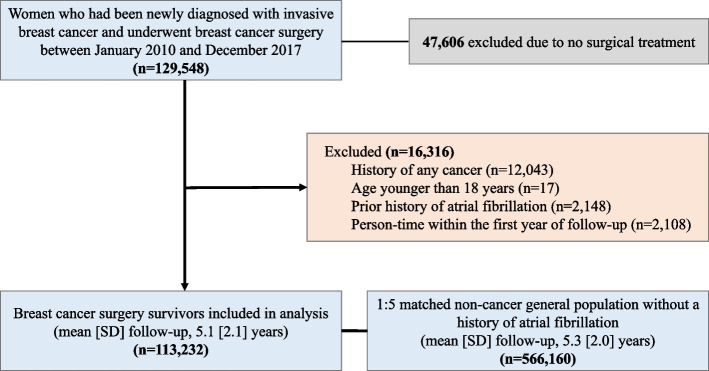
We identified 129,548 women newly diagnosed with invasive breast cancer who had undergone breast cancer surgery between January 1, 2010, and December 31, 2017, based on both International Classification of Diseases, Tenth Revision (ICD-10) codes (C50) and cancer-specific insurance claim code (V193 code). This V code was used to ensure the accuracy of breast cancer diagnosis because it is a reimbursement code representing biopsy-confirmed cancers in South Korea [23]. We excluded those who did not undergo breast cancer surgery to avoid late-stage or aggressive breast cancer cases. Additionally, we excluded those who had a history of any cancer (n = 12,043), were younger than 18 years old (n = 17), or had a prior history of AF (n = 2,148). We further excluded person-time within the first year of follow-up to reduce the bias related to undetected AF present at baseline and the acute effect of breast cancer treatments on AF risk [1] (n = 2108). The resulting 113,232 breast cancer survivors were matched 1:5 to noncancer female general population with no prior history of AF (n = 566,160) from the general population based on birth year at baseline. Data recorded through December 31, 2020, were included in our analyses (Fig. 1).
Fig. 1.
Participant flow diagram. After excluding participants meeting exclusion criteria, 113,232 subjects were included in the final analysis
Measurements
The primary outcome was incident AF, based on ICD-10 codes of I48.0–I48.4, and I48.9. AF diagnosis using ICD-10 codes in the NHIS has been shown to be 94.1% accurate [24]. We defined individuals with AF as those who had a discharge diagnosis or visited an outpatient clinic more than twice to exclude those with transient AF and improve diagnostic accuracy [14, 25].
Breast cancer treatment information was based on the claims data within 1 year after the breast cancer diagnosis [23, 26]. Chemotherapy was defined as at least 1 treatment cycle of a chemotherapeutic agent (anthracyclines [epirubicin or doxorubicin], cyclophosphamide, and taxane-based regimens [docetaxel or paclitaxel]). Due to the reimbursement policy in the NHIS, most patients administered taxane-based regimens tended to take anthracyclines in the adjuvant setting, indicating that those given taxanes could also be included in a category of those who took anthracyclines [26]. Targeted therapy was defined as at least one treatment cycle of Herceptin (trastuzumab). Endocrine therapy was defined as treatment with tamoxifen or aromatase inhibitors (anastrozole, exemestane, and letrozole), categorizing the use of a specific regimen based on the initial prescription when there was a subsequent switching between medicines. Radiation therapy was defined as at least one local or regional treatment.
Comorbid medical conditions were assessed using past medical history data and clinical and pharmacy ICD-10 codes. Comorbidities included hypertension, type 2 diabetes, dyslipidemia, coronary heart disease (CHD), congestive heart failure, stroke, chronic kidney disease, and chronic obstructive pulmonary disease (COPD). Comorbidities of participants were identified based on laboratory measures, claims, and prescription information prior to the index date as follows: hypertension (ICD-10 codes [I10.x-I13.x and I15.x], or being on antihypertensive medication or having blood pressure ≥ 140/90 mmHg), diabetes mellitus (DM) (ICD-10 codes [E11.x-E14.x] with antidiabetic medications, or a fasting glucose level ≥ 126 mg/dL), dyslipidemia (ICD-10 code E78.x with lipid-lowering medication, or total cholesterol level ≥ 240 mg/dL), coronary heart disease (ICD-10 codes [I21.x-I22.x] during hospitalization), congestive heart failure (ICD codes [I50.x] for the first hospitalization), stroke (ICD-10 codes [I63.x-I64.x] during hospitalization, with claims for brain magnetic resonance imaging or brain computed tomography), chronic kidney disease (the glomerular filtration rate of < 60 mL/min/1.73 m2 as estimated by the Modification of Diet in Renal Disease equation), and chronic obstructive pulmonary disease (ICD-10 codes [J43.x-J44.x]). ICD-10 codes were also applied if recorded in at least two outpatient visits for coronary heart disease and stroke. In addition, the Charlson Comorbidity Index (CCI) was calculated based on ICD-10 codes. Income level was based on monthly health insurance premiums. Low-income status was defined as being in the lowest quartile of monthly health insurance premiums or being enrolled in the Medical Aid program. The geographic area of residence was dichotomized by rural and urban areas using the primary local authority districts (shi/gun/gu).
Statistical analysis
The baseline characteristics are presented as the mean with standard deviations or numbers with percentages. Participant data were followed until the date of the first AF diagnosis, death, or end of the study, whichever occurred first. Person-time was calculated starting at 1 year after baseline. The crude AF incidence rate was assessed by dividing the number of events by the total number of person-years of follow-up presented as per 1000 person-years. Competing risk survival statistics considering death as a competing risk were used to calculate the cumulative incidence of AF [27]. The AF incidence between women with and without breast cancer was compared using the Gray-K test [28].
The Fine–Gray proportional sub-distribution hazards model was used to estimate sub-distribution hazard ratios (sHRs) and 95% confidence intervals (CIs) for AF incidence with death as a competing risk [29]. We applied the Fine–Gray model rather than the cause-specific hazards model because we aimed to evaluate the total effect of exposure (i.e., the presence of breast cancer in the total study population and cancer treatment in breast cancer survivors) on incident AF, not to determine the biological mechanism of the associations [30]. The proportional hazards assumption was assessed using Schoenfeld’s residuals, and no specific departure was observed. In addition to a crude model (Model 1), potential confounders were identified a priori based on a literature review; these included age at baseline (continuous variable), household income, and residential location, which were incorporated into Model 2 to account for sociodemographic characteristics in the association between the presence of breast cancer and AF risk. Model 3 further adjusted for the presence or absence of hypertension, type 2 diabetes, dyslipidemia, CHD, congestive heart failure, stroke, chronic kidney disease, and COPD to account for comorbidities. Then, we examined AF risk by different cancer treatment modalities, including anthracyclines, taxanes, trastuzumab, endocrine treatment, and radiation therapy among breast cancer survivors, further adjusting for the use of other cancer treatment modalities in Model 4. For instance, to examine the risk of AF, breast cancer survivors who underwent anthracycline treatment were compared with their counterparts who did not undergo anthracycline treatment. All analyses were stratified by age categories (18–39, 40–50, 51–65, or ≥ 66 years). Landmark analyses were performed using 2 landmark points (3 and 5 years after breast cancer diagnosis) to estimate AF risk in an unbiased way in individuals who were event-free at the landmark time and examine the long-term effects of breast cancer and its treatments on AF incidence [31].
As a sensitivity analysis, we included person-time within the first year of follow-up to capture any short-term CV consequences. In addition, using a subset of data comprising participants in the general health screening examination, we conducted sensitivity analyses among 72,560 women with and 292,468 without breast cancer to account for body mass index, smoking, alcohol consumption, and physical activity (see Additional file 1: Supplemental Methods for measurement details). A potential effect modification by income status, residential location, and other comorbidities was evaluated through stratified analysis and interaction testing using a likelihood ratio test. All statistical analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA). The P values provided are two-sided, with the level of significance at 0.05.
Results
Baseline characteristics
Baseline characteristics are shown in Table 1, stratified by the presence or absence of breast cancer. The mean study population age was 51.6 years, and 10.8% of participants were less than 40 years old. Individuals surgically treated for breast cancer (hereafter referred to as breast cancer survivors) were more likely to have hypertension, type 2 diabetes, dyslipidemia, CHD, congestive heart failure, chronic kidney disease, and COPD than women without cancer. Among breast cancer survivors, the proportions of use in each treatment option were 61.0% for chemotherapy, 15.0% for target therapy, 61.8% for endocrine treatment, and 69.8% for radiation therapy. Breast cancer survivors were less likely to have low incomes or live in rural area. Overall, the prevalence of CV risk factors and comorbidities was higher in breast cancer survivors than in women without cancer in each age group (Additional file 2: Table S1). In addition, women with breast cancer aged 40 or over had a higher prevalence of CV risk factors and comorbidities compared to those with breast cancer younger than 40 years (Additional file 2: Table S2).
Table 1.
Baseline characteristics of the study population and subgroup of general health examination participants
| Breast cancer | ||
|---|---|---|
| No (N = 566,160) | Yes (N = 113,232) | |
| Age at baseline, mean (SD), years | 51.6 (10.8) | 51.6 (10.8) |
| Age group, years | ||
| 18–39 | 61,325 (10.8) | 12,265 (10.8) |
| 40–50 | 202,435 (35.8) | 40,487 (35.8) |
| 51–65 | 235,670 (41.6) | 47,134 (41.6) |
| ≥ 66 | 66,730 (11.8) | 13,346 (11.8) |
| Comorbidity | ||
| Hypertension | 122,631 (21.7) | 28,779 (25.4) |
| Type 2 diabetes | 39,756 (7.0) | 10,027 (8.9) |
| Dyslipidemia | 100,734 (17.8) | 23,068 (20.4) |
| Coronary heart disease | 33,511 (5.9) | 8732 (7.7) |
| Congestive heart failure | 7670 (1.4) | 3046 (2.7) |
| Stroke | 12,177 (2.2) | 2464 (2.2) |
| Chronic kidney disease | 4444 (0.8) | 1285 (1.1) |
| Chronic obstructive pulmonary disease | 53,517 (9.5) | 20,698 (18.3) |
| Charlson comorbidity index, mean (SD) | 0.9 (1.2) | 2.5 (1.3) |
| Cancer treatment type | ||
| Chemotherapy, Yes | 69,056 (61.0) | |
| Anthracyclines | 58,709 (85.0) | |
| Cyclophosphamide | 65,140 (94.3) | |
| Fluorouracil | 15,801 (22.9) | |
| Taxane | 36,417 (52.7) | |
| Othersa | 7937 (11.5) | |
| Trastuzumab, Yes | 16,945 (15.0) | |
| Endocrine therapy, Yes | 77,925 (61.8) | |
| Tamoxifen | 49,574 (43.8) | |
| Aromatase inhibitors | 27,046 (23.9) | |
| Both | 1305 (1.2) | |
| Radiation therapy, Yes | 79,076 (69.8) | |
| Income status, Low | 140,488 (24.8) | 25,507 (22.5) |
| Residential location, Urban | 265,234 (46.8) | 56,703 (50.1) |
| General health examination participants | No (N = 292,468) | Yes (N = 72,560) |
| Smoking, Ever | 14,835 (5.1) | 4216 (5.8) |
| Alcohol consumption (≥ 10 g), Yes | 70,695 (24.2) | 17,531 (24.2) |
| Regular physical activityb, Yes | 54,062 (18.5) | 13,330 (18.4) |
| Body mass index, mean (SD), kg/m2 | 23.6 (3.3) | 23.6 (3.4) |
| Obesity (BMI ≥ 25 kg/m2) | 86,548 (29.6) | 22,264 (30.7) |
Data are expressed as number (%) or mean (standard deviation) unless otherwise noted
aMethotrexate or cisplatin
bRegular physical activity was defined as at least 30 min of moderate physical activity for ≥ 5 days weekly or at least 20 min of strenuous physical activity ≥ 2 days weekly
Risk of AF in breast cancer survivors compared to the general population by age group
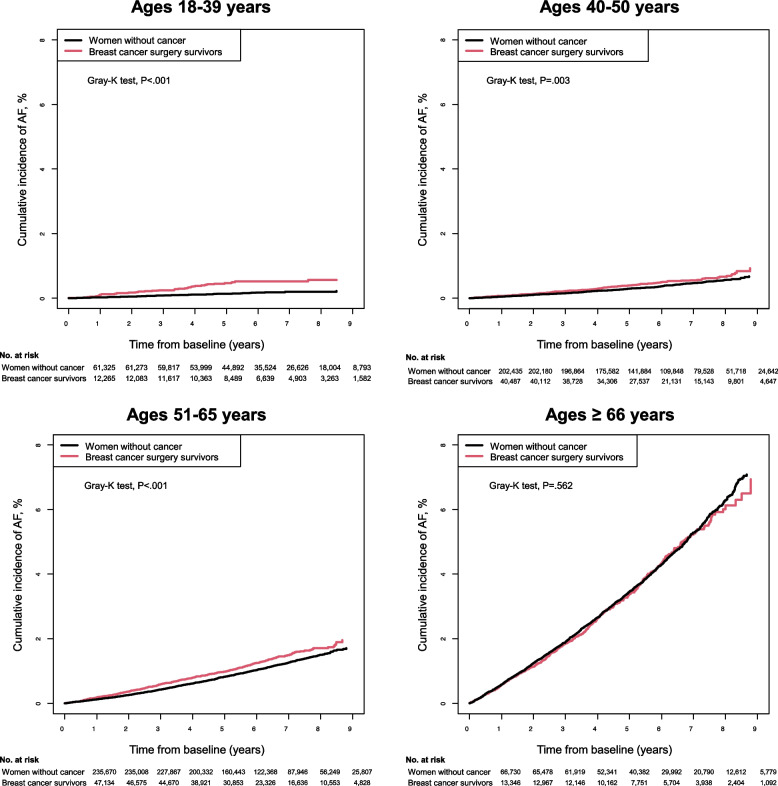
Risks of AF by age group are shown in Fig. 2 and Table 2. A total of 1166 (1.0%) incident AF cases were identified at least 1 year after baseline during a mean (SD) of 5.1 (2.1) years of follow-up in breast cancer survivors. Sub-distribution hazard (sub-distribution hazard is hereafter referred to as risk) of AF development was 6% higher in breast cancer survivors than those without cancer (Model 3: sHR 1.06, 95% CI 1.00–1.13) after adjusting for potential confounders. Associations differed by age groups (P for interaction < 0.001). Younger women (18–39 years) with breast cancer exhibited a 2.79-fold increased AF risk (sHR 2.79, 95% CI 1.98–3.94), whereas older women (≥ 66 years) with breast cancer exhibited a decreased AF risk (sHR 0.90, 95% CI 0.81–0.99), compared with those without cancer. In the landmark analysis, AF risk was attenuated over time, and there was no overall association with AF over 5 years after breast cancer diagnosis. However, younger women with breast cancer exhibited a sustained twofold increased risk in those aged < 40 years and at least a 30% increased AF risk in those aged ≤ 50 years during follow-up (Additional file 2: Table S3). In contrast, the inverse association tended to persist in those aged > 65 years (P for interaction for age group in both landmark analyses < 0.05).
Fig. 2.
Cumulative incidence of atrial fibrillation (AF) by age group. Cumulative incidence function plots for atrial fibrillation (AF) in breast cancer surgery survivors display that breast cancer surgery survivors had a consistently higher incidence of AF compared to their age-matched noncancer female general population in those aged 18–39 (a P < 0.001), aged 40–50 (b P = 0.003), and aged 51–65 (c P < 0.001)
Table 2.
Adjusted sub-distribution hazard ratios for developing atrial fibrillation in breast cancer surgery survivors compared to the noncancer general population by age categories
| Age group | Subjects (N) | Case (n) | IR per 1000 person-years | Model 1 (Crude) sHR (95% CI) |
Model 2 sHR (95% CI) |
Model 3 sHR (95% CI) |
||
|---|---|---|---|---|---|---|---|---|
| Main analysis | All ages | Noncancer | 566,160 | 5,271 | 1.76 | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) |
| Breast cancer | 113,232 | 1,166 | 2.01 | 1.14 (1.07–1.22) | 1.15 (1.08–1.23) | 1.06 (1.00–1.13) | ||
| 18–39 | Noncancer | 61,325 | 91 | 0.27 | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | |
| Breast cancer | 12,265 | 51 | 0.78 | 2.94 (2.09–4.15) | 2.95 (2.10–4.16) | 2.79 (1.98–3.94) | ||
| 40–50 | Noncancer | 202,435 | 684 | 0.63 | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | |
| Breast cancer | 40,487 | 171 | 0.81 | 1.28 (1.09–1.52) | 1.29 (1.09–1.53) | 1.22 (1.03–1.45) | ||
| 51–65 | Noncancer | 235,670 | 2,123 | 1.71 | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | |
| Breast cancer | 47,134 | 497 | 2.06 | 1.21 (1.10–1.33) | 1.22 (1.10–1.34) | 1.13 (1.03–1.25) | ||
| ≥ 66 | Noncancer | 66,730 | 2,373 | 7.31 | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | |
| Breast cancer | 13,346 | 447 | 7.08 | 0.97 (0.88–1.07) | 0.98 (0.89–1.09) | 0.90 (0.81–0.99) | ||
| P for interaction | < 0.001 | < 0.001 | < 0.001 | |||||
| 3–year landmark analysis | All ages | Noncancer | 546,453 | 3,609 | 1.94 | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) |
| Breast cancer | 107,153 | 777 | 2.17 | 1.12 (1.04–1.21) | 1.13 (1.05–1.22) | 1.05 (0.97–1.14) | ||
| 18–39 | Noncancer | 59,817 | 60 | 0.28 | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | |
| Breast cancer | 11,617 | 31 | 0.76 | 2.75 (1.78–4.24) | 2.76 (1.79–4.26) | 2.62 (1.70–4.05) | ||
| 40–50 | Noncancer | 196,863 | 472 | 0.69 | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | |
| Breast cancer | 38,727 | 117 | 0.89 | 1.28 (1.05–1.57) | 1.29 (1.06–1.58) | 1.23 (1.00–1.50) | ||
| 51–65 | Noncancer | 227,856 | 1,516 | 1.97 | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | |
| Breast cancer | 44,665 | 328 | 2.22 | 1.13 (1.00–1.27) | 1.14 (1.01–1.28) | 1.06 (0.94–1.20) | ||
| ≥ 66 | Noncancer | 61,917 | 1,561 | 8.04 | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | |
| Breast cancer | 12,144 | 301 | 8.06 | 1.00 (0.89–1.14) | 1.02 (0.90–1.15) | 0.93 (0.82–1.05) | ||
| P for interaction | < 0.001 | < 0.001 | < 0.001 | |||||
| 5-year landmark analysis | All ages | Noncancer | 546,453 | 1,918 | 2.11 | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) |
| Breast cancer | 107,153 | 388 | 2.25 | 1.07 (0.96–1.19) | 1.08 (0.96–1.19) | 1.00 (0.90–1.12) | ||
| 18–39 | Noncancer | 59,817 | 28 | 0.25 | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | |
| Breast cancer | 11,617 | 12 | 0.59 | 2.32 (1.18–4.56) | 2.33 (1.18–4.57) | 2.22 (1.13–4.37) | ||
| 40–50 | Noncancer | 196,863 | 265 | 0.79 | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | |
| Breast cancer | 38,727 | 65 | 1.01 | 1.28 (0.98–1.68) | 1.29 (0.98–1.69) | 1.23 (0.94–1.62) | ||
| 51–65 | Noncancer | 227,856 | 812 | 2.18 | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | |
| Breast cancer | 44,665 | 163 | 2.31 | 1.06 (0.90–1.25) | 1.06 (0.90–1.26) | 1.00 (0.84–1.18) | ||
| ≥ 66 | Noncancer | 61,917 | 813 | 9.08 | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | |
| Breast cancer | 12,144 | 148 | 8.70 | 0.96 (0.80–1.14) | 0.96 (0.81–1.15) | 0.89 (0.74–1.06) | ||
| P for interaction | 0.041 | 0.041 | 0.024 | |||||
Landmark analysis was conducted to estimate AF risk in individuals who were event-free at specific time points (landmark time), 3 and 5 years post-breast cancer diagnosis, respectively
Model 2: adjusted for age, income status, and residential location
Model 3: adjusted for Model 2 + hypertension, type 2 diabetes, dyslipidemia, coronary heart disease, congestive heart failure, chronic kidney disease, and chronic obstructive pulmonary disease
IR Incidence rate, PYs Person-years, sHR Sub-distribution hazard ratio, CI Confidence interval
Risk of AF by treatment modalities
Risks of AF by cancer treatment modalities among breast cancer survivors are shown in Table 3. Breast cancer survivors who received anthracyclines had a 57% higher AF risk compared with those who did not (Model 4: sHR 1.57, 95% CI 1.28–1.92), whereas tamoxifen users had a 19% lower AF risk compared with those who did not receive endocrine treatment (sHR 0.81, 95% CI 0.70–0.95). Breast cancer survivors who received taxane-based chemotherapy had increased AF risk compared with those who did not receive taxane-based chemotherapy (Model 3: sHR 1.20, 95% CI 1.06–1.35), but the association became nonsignificant after further adjusting for other cancer treatment modalities (Model 4: sHR 0.84, 95% CI 0.69–1.02). Other cancer therapies, including trastuzumab, aromatase inhibitors, and radiation treatment, were not associated with AF incidence among breast cancer survivors. In the landmark analyses, breast cancer survivors who used anthracyclines showed persistent increased AF risk over the follow-up period compared with those who did not use anthracyclines. In contrast, the inverse association between tamoxifen use and AF risk tended to disappear.
Table 3.
Adjusted sub-distribution hazard ratios for developing atrial fibrillation by cancer treatment type among breast cancer surgery survivors
| Treatment type | Subjects (N) | Case (n) | IR per 1000 PYs | Model 1 (Crude) sHR (95% CI) |
Model 2 sHR (95% CI) |
Model 3 sHR (95% CI) |
Model 4 sHR (95% CI) |
||
|---|---|---|---|---|---|---|---|---|---|
| Main analysis | Anthracyclines | No | 54,523 | 591 | 2.14 | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) |
| Yes | 58,709 | 575 | 1.89 | 0.88 (0.79–0.98) | 1.39 (1.23–1.58) | 1.39 (1.23–1.57) | 1.57 (1.28–1.92) | ||
| Taxane | No | 48,092 | 530 | 2.17 | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | |
| Yes | 65,140 | 636 | 1.89 | 0.87 (0.77–0.97) | 1.22 (1.08–1.37) | 1.20 (1.06–1.35) | 0.84 (0.69–1.02) | ||
| Trastuzumab | No | 96,287 | 996 | 2.00 | 1(Ref.) | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | |
| Yes | 16,945 | 170 | 2.04 | 1.03 (0.87–1.21) | 1.15 (0.97–1.35) | 1.11 (0.94–1.30) | 0.96 (0.80–1.14) | ||
| Endocrine therapy | No | 35,307 | 386 | 2.19 | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | |
| Tamoxifen | 49,574 | 294 | 1.12 | 0.51 (0.44–0.59) | 0.79 (0.68–0.92) | 0.78 (0.67–0.92) | 0.81 (0.70–0.95) | ||
| AIs | 27,046 | 471 | 3.46 | 1.58 (1.38–1.81) | 1.01 (0.88–1.15) | 1.00 (0.87–1.15) | 1.00 (0.87–1.15) | ||
| Both | 1305 | 15 | 2.23 | 1.02 (0.61–1.70) | 0.70 (0.42–1.17) | 0.66 (0.40–1.11) | 0.68 (0.41–1.14) | ||
| Radiation therapy | No | 34,156 | 433 | 2.52 | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | |
| Yes | 79,076 | 733 | 1.79 | 0.71 (0.63–0.80) | 1.01 (0.89–1.14) | 1.02 (0.90–1.16) | 0.98 (0.87–1.12) | ||
| 3-year landmark analysis | Anthracyclines | No | 52,036 | 390 | 2.32 | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) |
| Yes | 55,117 | 387 | 2.04 | 0.88 (0.76–1.01) | 1.41 (1.22–1.64) | 1.40 (1.21–1.63) | 1.48 (1.16–1.90) | ||
| Taxane | No | 45,695 | 345 | 2.31 | 1 (Ref.) | 1(Ref.) | 1 (Ref.) | 1 (Ref.) | |
| Yes | 61,458 | 432 | 2.07 | 0.90 (0.78–1.03) | 1.27 (1.10–1.48) | 1.25 (1.08–1.45) | 0.91 (0.72–1.16) | ||
| Trastuzumab | No | 91,190 | 663 | 2.15 | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | |
| Yes | 15,963 | 114 | 2.28 | 1.07 (0.88–1.30) | 1.19 (0.98–1.46) | 1.16 (0.95–1.41) | 0.99 (0.80–1.23) | ||
| Endocrine therapy | No | 32,452 | 252 | 2.34 | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | |
| Tamoxifen | 47,839 | 208 | 1.27 | 0.54 (0.45–0.65) | 0.85 (0.70–1.03) | 0.85 (0.70–1.02) | 0.89 (0.74–1.08) | ||
| AIs | 25,633 | 308 | 3.72 | 1.59 (1.35–1.88) | 0.99 (0.84–1.18) | 0.99 (0.83–1.17) | 0.99 (0.83–1.17) | ||
| Both | 1229 | 9 | 2.16 | 0.92 (0.47–1.79) | 0.62 (0.32–1.21) | 0.59 (0.30–1.14) | 0.61 (0.31–1.18) | ||
| Radiation therapy | No | 31,842 | 283 | 2.70 | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | |
| Yes | 75,311 | 494 | 1.95 | 0.72 (0.62–0.84) | 1.04 (0.89–1.21) | 1.05 (0.90–1.22) | 1.01 (0.87–1.18) | ||
| 5-year landmark analysis | Anthracyclines | No | 34,664 | 183 | 2.32 | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) |
| Yes | 39,965 | 205 | 2.20 | 0.95 (0.78–1.16) | 1.57 (1.27–1.94) | 1.56 (1.26–1.93) | 1.56 (1.10–2.21) | ||
| Taxane | No | 31,154 | 160 | 2.28 | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | |
| Yes | 43,475 | 228 | 2.24 | 0.98 (0.80–1.20) | 1.43 (1.16–1.76) | 1.40 (1.14–1.73) | 1.02 (0.72–1.43) | ||
| Trastuzumab | No | 64,089 | 332 | 2.22 | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | |
| Yes | 10,540 | 56 | 2.45 | 1.11 (0.84–1.47) | 1.24 (0.93–1.65) | 1.20 (0.90–1.59) | 1.01 (0.75–1.37) | ||
| Endocrine therapy | No | 22,325 | 115 | 2.23 | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | |
| Tamoxifen | 34,191 | 116 | 1.46 | 0.65 (0.50–0.84) | 1.04 (0.80–1.35) | 1.04 (0.80–1.35) | 1.13 (0.86–1.48) | ||
| AIs | 17,253 | 152 | 3.89 | 1.74 (1.37–2.22) | 1.07 (0.83–1.36) | 1.06 (0.83–1.36) | 1.08 (0.84–1.38) | ||
| Both | 860 | 5 | 2.49 | NA | NA | NA | NA | ||
| Radiation therapy | No | 21,464 | 144 | 2.86 | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | |
| Yes | 53,165 | 244 | 2.00 | 0.70 (0.57–0.86) | 1.02 (0.82–1.26) | 1.03 (0.83–1.28) | 0.98 (0.79–1.22) | ||
Model 2: adjusted for age, income status, and residential location
Model 3: adjusted for Model 2 + hypertension, type 2 diabetes, dyslipidemia, coronary heart disease, congestive heart failure, chronic kidney disease, and chronic obstructive pulmonary disease
Model 4: adjusted for Model 3 + use of anthracyclines, taxane, trastuzumab, endocrine therapy, and radiation therapy
IR Incidence rate, PYs Person-years, sHR Sub-distribution hazard ratio, CI Confidence interval, AIs Aromatase inhibitors, NA Not applicable
Risks of AF according to cancer treatment modalities and age group among breast cancer survivors are shown in Table 4. Breast cancer survivors who used anthracyclines had increased AF risk compared with those who did not use anthracyclines in all age groups, with a stronger association among younger women aged ≤ 50 years. The inverse association between tamoxifen use and AF risk also tended to be clearer among younger women aged ≤ 50 years (Additional file 2: Table S4). In the landmark analyses, the use of anthracyclines tended to be consistently associated with increased AF risk in each age group over the follow-up. In contrast, the inverse association between tamoxifen use and AF risk was no longer significant (Additional file 2: Table S5).
Table 4.
Adjusted sub-distribution hazard ratios for developing atrial fibrillation by cancer treatment type among breast cancer surgery survivors by age categories
| Treatment type | Age 18–39 | Age 40–50 | Age 51–65 | Age ≥ 66 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C/S | IR | sHR (95% CI) | C/S | IR | sHR (95% CI) | C/S | IR | sHR (95% CI) | C/S | IR | sHR (95% CI) | |
| Anthracyclines | ||||||||||||
| No | 9/4532 | 0.38 | 1 (Ref.) | 59/18,346 | 0.62 | 1 (Ref.) | 195/21,802 | 1.75 | 1 (Ref.) | 328/9843 | 7.07 | 1 (Ref.) |
| Yes | 42/7733 | 1.02 | 3.07 (1.47–6.42) | 112 /22,141 | 0.95 | 1.69 (1.18–2.40) | 302/25,332 | 2.33 | 1.53 (1.20–1.93) | 119/3503 | 7.10 | 1.51 (1.15–1.98) |
| Taxane | ||||||||||||
| No | 15/4426 | 0.64 | 1 (Ref.) | 56/16,694 | 0.65 | 1 (Ref.) | 170/18,796 | 1.77 | 1 (Ref.) | 289/8176 | 7.47 | 1 (Ref.) |
| Yes | 36/7839 | 0.86 | 0.92 (0.49–1.71) | 115/23,793 | 0.92 | 0.93 (0.65–1.33) | 327/28,338 | 2.26 | 0.85 (0.67–1.09) | 158/5170 | 6.45 | 0.81 (0.63–1.03) |
| Trastuzumab | ||||||||||||
| No | 44/10,465 | 0.78 | 1 (Ref.) | 142/35,100 | 0.77 | 1 (Ref.) | 410/38,765 | 2.05 | 1 (Ref.) | 400/11,957 | 7.02 | 1 (Ref.) |
| Yes | 7/1800 | 0.78 | 0.85 (0.38–1.89) | 29/5387 | 1.08 | 1.16 (0.78–1.74) | 87/8369 | 2.12 | 0.88 (0.69–1.12) | 47/1389 | 7.57 | 1.05 (0.77–1.44) |
| Endocrine therapy | ||||||||||||
| No | 24/4462 | 1.04 | 1 (Ref.) | 53/10,629 | 0.99 | 1 (Ref.) | 168/15,998 | 2.10 | 1 (Ref.) | 141/4218 | 7.34 | 1 (Ref.) |
| Tamoxifen | 27/7741 | 0.65 | 0.59 (0.34–1.02) | 106/28,566 | 0.70 | 0.72 (0.52–1.00) | 91/11,024 | 1.57 | 0.94 (0.72–1.21) | 70/2243 | 6.32 | 0.82 (0.61–1.09) |
| AIs | 0/35 | 0 | NA | 8/1149 | 1.27 | 1.01 (0.48–2.13) | 233/19,253 | 2.37 | 1.04 (0.85–1.27) | 230/6609 | 7.30 | 1.03 (0.84–1.27) |
| Both | 0/27 | 0 | NA | 4/143 | 5.15 | NA | 5/859 | 1.12 | NA | 6/276 | 4.40 | 0.57 (0.25–1.28) |
| Radiation therapy | ||||||||||||
| No | 12/3485 | 0.67 | 1 (Ref.) | 42/11,160 | 0.73 | 1 (Ref.) | 144/13,091 | 2.17 | 1 (Ref.) | 235/6420 | 7.81 | 1 (Ref.) |
| Yes | 39/8780 | 0.83 | 1.18 (0.62–2.25) | 129/29,327 | 0.83 | 1.11 (0.79–1.58) | 353/34,043 | 2.02 | 0.96 (0.79–1.17) | 212/6926 | 6.41 | 0.99 (0.82–1.20) |
Hazard ratios were adjusted for age, income status, residential location, hypertension, type 2 diabetes, dyslipidemia, coronary heart disease, congestive heart failure, chronic kidney disease, chronic obstructive pulmonary disease, and history of anthracyclines, taxane, trastuzumab, endocrine therapy, and radiation therapy
C/S The number of cases/the number of subjects, IR Incidence rate (described as per 1000 person-years), sHR Sub-distribution hazard ratio, CI Confidence interval, AIs Aromatase inhibitors, NA Not applicable
Stratified analyses and sensitivity analyses
In stratified analyses, increased AF risk was observed only for those without CHD (P for interaction = 0.04) or those without COPD (P for interaction = 0.06) among women with breast cancer of all ages (Additional file 2: Table S6). When we included person-time within the first year of follow-up, the associations tended to be stronger, particularly in younger breast cancer survivors (aged ≤ 50 years) (Additional file 2: Table S7). In contrast, meaningful changes were not observed in risks of AF by cancer treatment modalities among breast cancer survivors (Additional file 2: Table S8). The results were not materially changed in analyses limited to participants in the NHIS health examinations where body mass index, smoking, alcohol consumption, and physical activity were further adjusted for (Additional file 2: Tables S9 and S10).
Discussion
In this nationwide population-based cohort study, younger breast cancer survivors had increased AF risk compared to an age-matched sample of females with no cancer history from the general population; the strength of this association remained persistent over 5 years of follow-up. Breast cancer survivors aged < 40 years had a more than twofold increased AF risk during follow-up; this elevated risk was not observed in older breast cancer survivors, particularly those aged > 65 years. Among breast cancer survivors, those treated with anthracyclines had a more pronounced AF risk than those not exposed to this chemotherapeutic. The association was strongest in breast cancer survivors aged ≤ 50 years, and remained persistent over the follow-up period.
Newly developed AF in individuals with cancer may have an adverse effect on prognosis. In a study using the US Surveillance, Epidemiology, and End Results Medicare registry data, old women aged > 65 years with breast cancer who developed incident AF within the first 30 days of breast cancer diagnosis were 3 times more likely to die of a CV condition within 1 year compared with those without incident AF [6]. In a Taiwanese retrospective cohort study, individuals with cancer who developed new-onset AF had an increased risk of thromboembolism and heart failure, although all-cause mortality was not elevated [32]. In contrast, new-onset AF in those with lymphoma was positively associated with both acute heart failure and all-cause mortality [33]. In addition, there was a higher risk of bleeding in patients with cancer and AF than in those without AF [34].
While we initially observed an overall positive association between breast cancer and the risk of developing AF, it was marginal and became null over the follow-up period. These results are similar to previous studies, which were unable to show a strong association between breast cancer and incident AF compared to other types of cancer [13, 14]. The strength of the association with the risk of incident AF also decreased over time, with the highest risk occurring in the first year of diagnosis [6, 7, 11, 13, 14], which was also observed in our study. This transient, increased risk of developing AF shortly after breast cancer diagnosis might be due to surgical and/or medical cancer therapy, autonomic nervous system imbalance, pre-existing chronic inflammatory state, cancer-related comorbidities, or the combination of these conditions [1]. To assess the mid-to-long-term risk of AF among breast cancer survivors, we excluded AF incidence in the first year of follow-up to mitigate the effects of these potential confounding factors.
In our study, younger survivors particularly aged < 40 years showed a stronger association with incident AF than older breast cancer survivors. The increased risk remained persistent over 5 years of the follow-up after adjusting for cardiometabolic comorbidities. A previous study reported that breast cancer survivors < 60 years had a stronger association with incident AF than older breast cancer survivors [8]. It is unclear why the association differs by age group. In our stratified analyses, among those without CV risk factors or CVD at baseline, breast cancer survivors had a higher AF risk compared to the general population. This suggests that AF incidence between older breast cancer survivors and the general population might not be different due to the increased prevalence of CV risk factors and comorbid CVD as women age. However, the prevalence of CV risk factors and comorbidities in women aged > 40 years was still higher in breast cancer survivors compared to the general population in our study. The other speculation is that younger patients receive more intensive, cardiotoxic treatments than older patients [8]. Tanenbaum et al. reported that younger cancer survivors are more likely to undergo screening for cardiovascular risk factors, compared to younger people without cancer [35]. However, given our findings of the overall 1% incidence of AF in this study cohort, the low incidence rate demands careful interpretation for clinical implications. Regular cardiac surveillance or monitoring for AF may be warranted, although further research is needed to establish a specific prevention strategy [36].
We observed a positive association between the use of anthracyclines and subsequent increased AF risk after accounting for use of other cancer treatment modalities. After adjusting for other cancer treatment modalities, there was no significant association between the use of taxanes and AF occurrence, suggesting that the observed unadjusted association was likely driven by the concomitant use of anthracyclines [26]. This is consistent with prior findings from a recent study of the World Health Organization dataset VigiBase of more than 130 countries, which reported that those who developed AF were more likely to use anthracyclines, adjusting for the use of other anticancer medications [37]. In a meta-analysis conducted in 2013, subclinical manifestations of cardiac toxicity were identified in 18% of the patients treated with an anthracycline after an average follow-up of 9 years, which was higher compared to 6% developed clinical cardiac toxicity [38]. Patients with more aggressive forms of breast cancer, such as triple-negative breast cancer, might be more likely to receive anthracyclines, which could make them more susceptible to AF [39]. Anthracyclines, such as doxorubicin, are known for their cardiotoxic effects, primarily due to the generation of reactive oxygen species leading to oxidative stress and myocardial damage [40]. This oxidative stress can trigger structural and electrical remodeling of the atrial myocardium, which is a known substrate for AF development [41]. Moreover, anthracyclines can induce cellular apoptosis and disrupt cardiac calcium metabolism, further contributing to AF incidence [42]. In contrast, our findings differ from those of previous studies from the US [6] and Canada [11], where anthracycline use was not associated with increased AF risk, possibly because of different study population characteristics such as racial/ethnic differences [43]. However, racial/ethnic differences in cardiotoxicity is understudied and further studies are warranted to confirm the observed finding [44]. Moreover, younger patients or those with fewer pre-existing cardiac risk factors might be preferentially selected for these treatments, impacting the observed association between anthracyclines and the incidence of AF. An inverse association between tamoxifen use and AF risk among younger breast cancer survivors aged ≤ 50 years tended to diminish gradually with increasing follow-up duration. This finding suggests that benefit of tamoxifen use may be limited to reduced risk of early CV outcomes in breast cancer survivors [45].
Given the limitations of claims data, we could not provide clinical details, including cancer stage, cardiac imaging, pathological results, surgery type, and chemotherapy dosage. In addition, misclassification and unmeasured confounding may exist as a result of limitations inherent to claims data. However, we investigated the mid- to long-term risk of AF after a breast cancer diagnosis and its surgical/medical treatments using data from a large sample size of 113,232 women newly diagnosed with breast cancer. We did not include some established AF risk factors such as obesity and lifestyle characteristics in our main analysis. However, we did perform various sensitivity analyses including further adjustment for body mass index, smoking, alcohol consumption, and physical activity to address this issue. In addition, there might be false positive findings due to a lack of correction for multiple comparisons. By employing the Fine–Gray model, our sub-distribution hazard ratio estimates may exhibit biases when compared to cause-specific hazard ratios [30]. Lastly, our definition of AF, requiring at least two diagnoses of AF for inclusion, may bias our outcome towards more severe cases, potentially underrepresenting milder or transient forms of this condition.
Conclusions
In conclusion, young breast cancer surgery survivors, specifically those aged < 40 years and those treated with anthracyclines, may be associated with increased mid- to long-term risk of AF. Our findings underscore the need for increased awareness of AF risk in this population.
Supplementary Information
Additional file 1. Supplementary Methods.
Additional file 2: Table S1. Baseline cardiovascular risk and comorbidities by age. Table S2. Cardiovascular risk factors in < 40 vs. ≥ 40 age groups. Table S3. Hazard ratios for AF in young breast cancer survivors. Table S4. AF risk by treatment in young survivors. Table S5. AF risk by age and treatment at 3- and 5-year marks. Table S6. AF risk in survivors vs. general population by factors. Table S7. Sensitivity analysis of AF risk by age after 1 year. Table S8. Treatment-based AF risk by age after 1 year. Table S9. AF risk in survivors vs. general population: Health screening subset. Table S10. Treatment-based AF risk: Health screening subset.
Acknowledgements
None.
Abbreviations
- AF
Atrial fibrillation
- CHD
Coronary heart disease
- CI
Confidence interval
- COPD
Chronic obstructive pulmonary disease
- CV
Cardiovascular
- DM
Diabetes mellitus
- NHIS
National Health Insurance Service
- SD
Standard deviation
- sHR
Sub-distribution hazard ratio
Authors’ contributions
All authors (Y-MP, WJ, YY, SHP, MGF, SJM, TT, VR, HSL, TZN, RSH-T, JLM, MS, BCA, KH, DWS) contributed to the study conception and design. Material preparation, data collection, and analysis were performed by SHP and KH. The first draft of the manuscript was written by Y-MP and WJ, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Authors’ Twitter handles
@DrJung_FM (Wonyoung Jung)
Funding
This research was partly supported by a grant from the Arkansas Breast Cancer Research Program.
Availability of data and materials
The data will be made available upon request and approval of a proposal by the National Health Insurance Service Database.
Declarations
Ethics approval and consent to participate
This study was approved by the Samsung Medical Center (Seoul, South Korea; SMC 2020-03-108) Institutional Review Board. All information used for analyses was anonymized and de-identified; therefore, informed consent was not required.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yong-Moon Mark Park and Wonyoung Jung contributed equally to this work.
Contributor Information
Kyungdo Han, Email: hkd917@naver.com.
Dong Wook Shin, Email: dwshin.md@gmail.com.
References
- 1.Farmakis D, Parissis J, Filippatos G. Insights into onco-cardiology: atrial fibrillation in cancer. J Am Coll Cardiol. 2014;63(10):945–953. doi: 10.1016/j.jacc.2013.11.026. [DOI] [PubMed] [Google Scholar]
- 2.Mehta LS, Watson KE, Barac A, Beckie TM, Bittner V, Cruz-Flores S, Dent S, Kondapalli L, Ky B, Okwuosa T, et al. Cardiovascular disease and breast cancer: where these entities intersect: a scientific statement from the American Heart Association. Circulation. 2018;137(8):e30–e66. doi: 10.1161/CIR.0000000000000556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Merino JL. Atrial fibrillation and breast cancer: casual or causal relationship? Eur Heart J. 2022;43(4):313–315. doi: 10.1093/eurheartj/ehab807. [DOI] [PubMed] [Google Scholar]
- 4.Miller KD, Nogueira L, Mariotto AB, Rowland JH, Yabroff KR, Alfano CM, Jemal A, Kramer JL, Siegel RL. Cancer treatment and survivorship statistics, 2019. CA Cancer J Clin. 2019;69(5):363–385. doi: 10.3322/caac.21565. [DOI] [PubMed] [Google Scholar]
- 5.Mauro E, Lucà F, Tetta C, Parise O, Parrini I, Parise G, Rao CM, Matteucci F, Micali LR, Gulizia MM, et al. Breast cancer and atrial fibrillation. J Clin Med. 2022;11(5):1417. doi: 10.3390/jcm11051417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guha A, Fradley MG, Dent SF, Weintraub NL, Lustberg MB, Alonso A, Addison D. Incidence, risk factors, and mortality of atrial fibrillation in breast cancer: a SEER-Medicare analysis. Eur Heart J. 2022;43(4):300–312. doi: 10.1093/eurheartj/ehab745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matthews AA, Peacock Hinton S, Stanway S, Lyon AR, Smeeth L, Bhaskaran K, Lund JL. Risk of cardiovascular diseases among older breast cancer survivors in the United States: a matched cohort study. J Natl Compr Canc Netw. 2021:1–10. https://pubmed.ncbi.nlm.nih.gov/33401236/, 10.6004/jnccn.2020.7629. [DOI] [PubMed]
- 8.D’Souza M, Smedegaard L, Madelaire C, Nielsen D, Torp-Pedersen C, Gislason G, Schou M, Fosbøl E. Incidence of atrial fibrillation in conjunction with breast cancer. Heart Rhythm. 2019;16(3):343–348. doi: 10.1016/j.hrthm.2018.10.017. [DOI] [PubMed] [Google Scholar]
- 9.Conen D, Wong JA, Sandhu RK, Cook NR, Lee IM, Buring JE, Albert CM. Risk of malignant cancer among women with new-onset atrial fibrillation. JAMA Cardiol. 2016;1(4):389–396. doi: 10.1001/jamacardio.2016.0280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O’Neal WT, Lakoski SG, Qureshi W, Judd SE, Howard G, Howard VJ, Cushman M, Soliman EZ. Relation between cancer and atrial fibrillation (from the REasons for Geographic And Racial Differences in Stroke Study) Am J Cardiol. 2015;115(8):1090–1094. doi: 10.1016/j.amjcard.2015.01.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abdel-Qadir H, Thavendiranathan P, Fung K, Amir E, Austin PC, Anderson GS, Lee DS. Association of early-stage breast cancer and subsequent chemotherapy with risk of atrial fibrillation. JAMA Netw Open. 2019;2(9):e1911838. doi: 10.1001/jamanetworkopen.2019.11838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saliba W, Rennert HS, Gronich N, Gruber SB, Rennert G. Association of atrial fibrillation and cancer: analysis from two large population-based case-control studies. PLoS One. 2018;13(1):e0190324. doi: 10.1371/journal.pone.0190324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jakobsen CB, Lamberts M, Carlson N, Lock-Hansen M, Torp-Pedersen C, Gislason GH, Schou M. Incidence of atrial fibrillation in different major cancer subtypes: a nationwide population-based 12 year follow up study. BMC Cancer. 2019;19(1):1105. doi: 10.1186/s12885-019-6314-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yun JP, Choi EK, Han KD, Park SH, Jung JH, Park SH, Ahn HJ, Lim JH, Lee SR, Oh S. Risk of atrial fibrillation according to cancer type: a nationwide population-based study. JACC CardioOncol. 2021;3(2):221–232. doi: 10.1016/j.jaccao.2021.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chao C, Xu L, Bhatia S, Cooper R, Brar S, Wong FL, Armenian SH. Cardiovascular disease risk profiles in survivors of adolescent and young adult (AYA) cancer: the Kaiser Permanente AYA Cancer Survivors Study. J Clin Oncol. 2016;34(14):1626–1633. doi: 10.1200/JCO.2015.65.5845. [DOI] [PubMed] [Google Scholar]
- 16.Boekel NB, Schaapveld M, Gietema JA, Russell NS, Poortmans P, Theuws JC, Schinagl DA, Rietveld DH, Versteegh MI, Visser O, et al. Cardiovascular disease risk in a large, population-based cohort of breast cancer survivors. Int J Radiat Oncol Biol Phys. 2016;94(5):1061–1072. doi: 10.1016/j.ijrobp.2015.11.040. [DOI] [PubMed] [Google Scholar]
- 17.Leong SP, Shen ZZ, Liu TJ, Agarwal G, Tajima T, Paik NS, Sandelin K, Derossis A, Cody H, Foulkes WD. Is breast cancer the same disease in Asian and Western countries? World J Surg. 2010;34(10):2308–2324. doi: 10.1007/s00268-010-0683-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee YH, Han K, Ko SH, Ko KS, Lee KU. Data analytic process of a nationwide population-based study using national health information database established by National Health Insurance Service. Diabetes Metab J. 2016;40(1):79–82. doi: 10.4093/dmj.2016.40.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheol Seong S, Kim YY, Khang YH, Heon Park J, Kang HJ, Lee H, Do CH, Song JS, Hyon Bang J, Ha S, et al. Data resource profile: the national health information database of the National Health Insurance Service in South Korea. Int J Epidemiol. 2017;46(3):799–800. doi: 10.1093/ije/dyw253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shin DW, Cho J, Park JH, Cho B. National General Health Screening Program in Korea: history, current status, and future direction. Precis Future Med. 2022;6(1):9–31. doi: 10.23838/pfm.2021.00135. [DOI] [Google Scholar]
- 21.Seo HJ, Oh IH, Yoon SJ. A comparison of the cancer incidence rates between the national cancer registry and insurance claims data in Korea. Asian Pac J Cancer Prev. 2012;13(12):6163–6168. doi: 10.7314/APJCP.2012.13.12.6163. [DOI] [PubMed] [Google Scholar]
- 22.Lee CK, Ha HJ, Oh SJ, Kim JW, Lee JK, Kim HS, Yoon SM, Kang SB, Kim ES, Kim TO, et al. Nationwide validation study of diagnostic algorithms for inflammatory bowel disease in Korean National Health Insurance Service database. J Gastroenterol Hepatol. 2020;35(5):760–768. doi: 10.1111/jgh.14855. [DOI] [PubMed] [Google Scholar]
- 23.Chung IY, Lee J, Park S, Lee JW, Youn HJ, Hong JH, Hur H. Nationwide analysis of treatment patterns for Korean Breast cancer survivors using National Health Insurance Service data. J Korean Med Sci. 2018;33(44):e276. doi: 10.3346/jkms.2018.33.e276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Choi EK. Cardiovascular research using the Korean National Health Information Database. Korean Circ J. 2020;50(9):754–772. doi: 10.4070/kcj.2020.0171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee SS, Ae Kong K, Kim D, Lim Y-M, Yang P-S, Yi J-E, Kim M, Kwon K, Bum Pyun W, Joung B, et al. Clinical implication of an impaired fasting glucose and prehypertension related to new onset atrial fibrillation in a healthy Asian population without underlying disease: a nationwide cohort study in Korea. Eur Heart J. 2017;38(34):2599–2607. doi: 10.1093/eurheartj/ehx316. [DOI] [PubMed] [Google Scholar]
- 26.Lee J, Hur H, Lee JW, Youn HJ, Han K, Kim NW, Jung SY, Kim Z, Kim KS, Lee MH, et al. Long-term risk of congestive heart failure in younger breast cancer survivors: a nationwide study by the SMARTSHIP group. Cancer. 2020;126(1):181–188. doi: 10.1002/cncr.32485. [DOI] [PubMed] [Google Scholar]
- 27.Kim HT. Cumulative incidence in competing risks data and competing risks regression analysis. Clin Cancer Res. 2007;13(2):559–565. doi: 10.1158/1078-0432.CCR-06-1210. [DOI] [PubMed] [Google Scholar]
- 28.Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988:1141–54. https://www.jstor.org/stable/2241622.
- 29.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94(446):496–509. doi: 10.1080/01621459.1999.10474144. [DOI] [Google Scholar]
- 30.Mansournia MA, Nazemipour M, Etminan M. A practical guide to handling competing events in etiologic time-to-event studies. Glob Epidemiol. 2022;4:100080. doi: 10.1016/j.gloepi.2022.100080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dafni U. Landmark analysis at the 25-year landmark point. Circ Cardiovasc Qual Outcomes. 2011;4(3):363–371. doi: 10.1161/CIRCOUTCOMES.110.957951. [DOI] [PubMed] [Google Scholar]
- 32.Hu Y-F, Liu C-J, Chang PM-H, Tsao H-M, Lin Y-J, Chang S-L, Lo L-W, Tuan T-C, Li C-H, Chao T-F. Incident thromboembolism and heart failure associated with new-onset atrial fibrillation in cancer patients. Int J Cardiol. 2013;165(2):355–357. doi: 10.1016/j.ijcard.2012.08.036. [DOI] [PubMed] [Google Scholar]
- 33.Amioka M, Sairaku A, Ochi T, Okada T, Asaoku H, Kyo T, Kihara Y. Prognostic significance of new-onset atrial fibrillation in patients with non-Hodgkin’s lymphoma treated with anthracyclines. Am J Cardiol. 2016;118(9):1386–1389. doi: 10.1016/j.amjcard.2016.07.049. [DOI] [PubMed] [Google Scholar]
- 34.Melloni C, Shrader P, Carver J, Piccini JP, Thomas L, Fonarow GC, Ansell J, Gersh B, Go AS, Hylek E, et al. Management and outcomes of patients with atrial fibrillation and a history of cancer: the ORBIT-AF registry. Eur Heart J Qual Care Clin Outcomes. 2017;3(3):192–197. doi: 10.1093/ehjqcco/qcx004. [DOI] [PubMed] [Google Scholar]
- 35.Tanenbaum HC, Xu L, Hahn EE, Wolfson J, Bhatia S, Cannavale K, Cooper R, Chao C. Preventive health service use among survivors of adolescent and young adult cancer. Prev Med Rep. 2020;20:101278. doi: 10.1016/j.pmedr.2020.101278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Madnick DL, Fradley MG. Atrial fibrillation and cancer patients: mechanisms and management. Curr Cardiol Rep. 2022;24(10):1517–1527. doi: 10.1007/s11886-022-01769-3. [DOI] [PubMed] [Google Scholar]
- 37.Alexandre J, Salem JE, Moslehi J, Sassier M, Ropert C, Cautela J, Thuny F, Ederhy S, Cohen A, Damaj G, et al. Identification of anticancer drugs associated with atrial fibrillation: analysis of the WHO pharmacovigilance database. Eur Heart J Cardiovasc Pharmacother. 2021;7(4):312–320. doi: 10.1093/ehjcvp/pvaa037. [DOI] [PubMed] [Google Scholar]
- 38.Lotrionte M, Biondi-Zoccai G, Abbate A, Lanzetta G, D’Ascenzo F, Malavasi V, Peruzzi M, Frati G, Palazzoni G. Review and meta-analysis of incidence and clinical predictors of anthracycline cardiotoxicity. Am J Cardiol. 2013;112(12):1980–1984. doi: 10.1016/j.amjcard.2013.08.026. [DOI] [PubMed] [Google Scholar]
- 39.Shah AN, Gradishar WJ. Adjuvant anthracyclines in breast cancer: what is their role? Oncologist. 2018;23(10):1153–1161. doi: 10.1634/theoncologist.2017-0672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huang J, Wu R, Chen L, Yang Z, Yan D, Li M. Understanding anthracycline cardiotoxicity from mitochondrial aspect. Front Pharmacol. 2022;13:811406. doi: 10.3389/fphar.2022.811406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tan R, Cong T, Xu G, Hao Z, Liao J, Xie Y, Lin Y, Yang X, Li Q, Liu Y, et al. Anthracycline-induced atrial structural and electrical remodeling characterizes early cardiotoxicity and contributes to atrial conductive instability and dysfunction. Antioxid Redox Signal. 2022;37(1–3):19–39. doi: 10.1089/ars.2021.0002. [DOI] [PubMed] [Google Scholar]
- 42.Burashnikov A. Atrial fibrillation induced by anticancer drugs and underling mechanisms. J Cardiovasc Pharmacol. 2022;80(4):540–546. doi: 10.1097/FJC.0000000000001182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang L, Song J, Clark R, Mangoni A, Goldberg Y, Slipczuk L, Garcia MJ, Bansal N, Sadler DB, Neilan T. Racial and ethnic differences in anthracycline cardiotoxicity. Circulation. 2021;144(Suppl_1):A13090. [Google Scholar]
- 44.Prasad P, Branch M, Asemota D, Elsayed R, Addison D, Brown S-A. Cardio-oncology preventive care: racial and ethnic disparities. Curr Cardiovasc Risk Rep. 2020;14(10):1–14. doi: 10.1007/s12170-020-00650-8. [DOI] [Google Scholar]
- 45.Rosell J, Nordenskjöld B, Bengtsson NO, Fornander T, Hatschek T, Lindman H, Malmström PO, Wallgren A, Stål O, Carstensen J. Effects of adjuvant tamoxifen therapy on cardiac disease: results from a randomized trial with long-term follow-up. Breast Cancer Res Treat. 2013;138(2):467–473. doi: 10.1007/s10549-013-2457-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Supplementary Methods.
Additional file 2: Table S1. Baseline cardiovascular risk and comorbidities by age. Table S2. Cardiovascular risk factors in < 40 vs. ≥ 40 age groups. Table S3. Hazard ratios for AF in young breast cancer survivors. Table S4. AF risk by treatment in young survivors. Table S5. AF risk by age and treatment at 3- and 5-year marks. Table S6. AF risk in survivors vs. general population by factors. Table S7. Sensitivity analysis of AF risk by age after 1 year. Table S8. Treatment-based AF risk by age after 1 year. Table S9. AF risk in survivors vs. general population: Health screening subset. Table S10. Treatment-based AF risk: Health screening subset.
Data Availability Statement
The data will be made available upon request and approval of a proposal by the National Health Insurance Service Database.