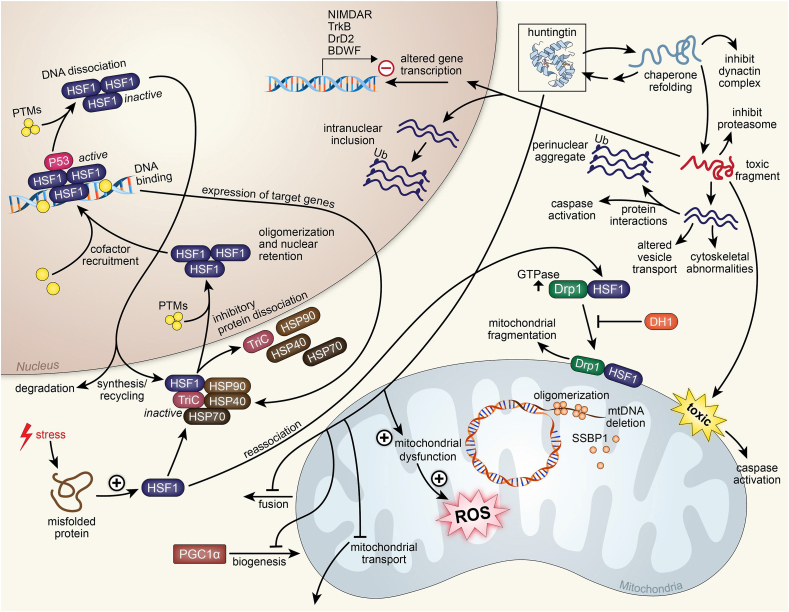
Fig. 4.
Huntington's disease.
Above is a figure illustrating mitochondrial affecting pathways in Huntington's disease associated with huntingtin and HSF1. Huntingtin in the mitochondria leads to mitochondrial dysfunction, leading to the production of ROS. Huntingtin also inhibits mitochondrial transport, inhibits PGC1α mitochondrial biogenesis, and inhibits mitochondrial fusion. With chaperone refolding, huntingtin can inhibit the dynactin complex. This refold can also become a toxic fragment that can inhibit the proteasome, and is also toxic to the mitochondria, leading to caspase activation. The toxic fragment can enter the mitochondria and negatively alter gene transcription, specifically affecting genes NIMDAR, TrkB, DrD2, and BDWF. In the nucleus, the toxic fragment can further associate with more fragments and create an intranuclear inclusion. The association of multiple toxic fragments can lead to cytoskeletal abnormalities and altered vesicle transport. These fragments can interact with proteins and lead to caspase activation. The association of more toxic fragments creates a perinuclear aggregate. Stress in the cell causing a misfolded protein causes the increase of HSF1. HSF1 can reassociate with Drp1 and in high GTPase conditions associate with mitochondria and lead to fragmentation. HSF1 with DRP1 allows mtDNA deletion by SSBP1. DH1 inhibits this process. HSF1 can form an inactive complex with proteins TriC, HSP70, HSP40, and HSP90 that allows it to travel into the nucleus where it dissociates with that complex and oligomerizes with other HSF1 proteins and is modified with PTMs for nuclear retention. With cofactors and P53, the HSF1 oligomer actively binds to DNA and expresses target genes TriC, HSP70, HSP40, and HSP90. Inhibitory PTMs allow the HSF1 oligomer to dissociate from DNA where it is either degraded in the cytoplasm or recycled for further synthesis of target genes.