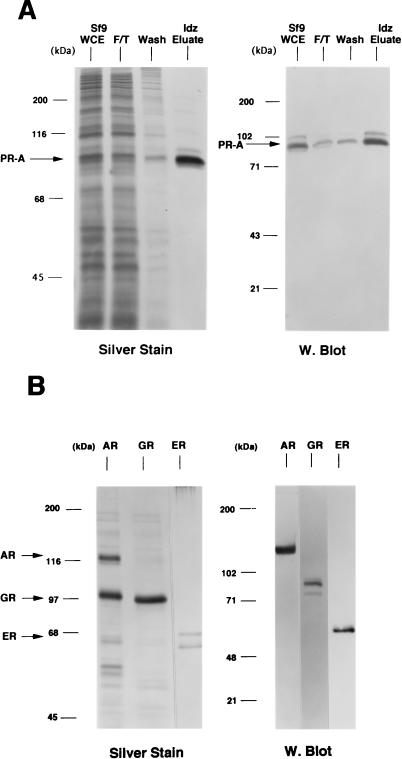
FIG. 1.
Purification of baculovirus-expressed steroid receptors. Human PR-A, AR, and GR were expressed as polyhistidine-tagged proteins in baculovirus and were purified by metal ion affinity resins (Talon). Human ER was expressed as a nonfusion protein in baculovirus and purified by using a DNA affinity column. (A) (Left panel) Silver-stained SDS–7.5% PAGE of PR-A purification fractions. Lanes: 1, Sf9 whole-cell extract (WCE; 5 μl); 2, Talon resin flowthrough (F/T; 5 μl); 3, column wash (Wash; 50 μl); and 4, 100 mM imidazole (Idz) eluate (10 μl). (Right panel) Western blot of the same PR-A purification fractions (5 μl) with the AB-52 MAb. (B) (Left panel) Silver-stained SDS-PAGE of purified AR and GR eluted from Talon resins by 100 mM imidazole. ER is an NaCl eluate of a DNA affinity resin. (Right panel) Western blots of purified AR, GR, and ER were done with the following receptor specific antibodies: N441, an MAb to AR; a polyclonal antibody to GR; and h151, an MAb to human ER.