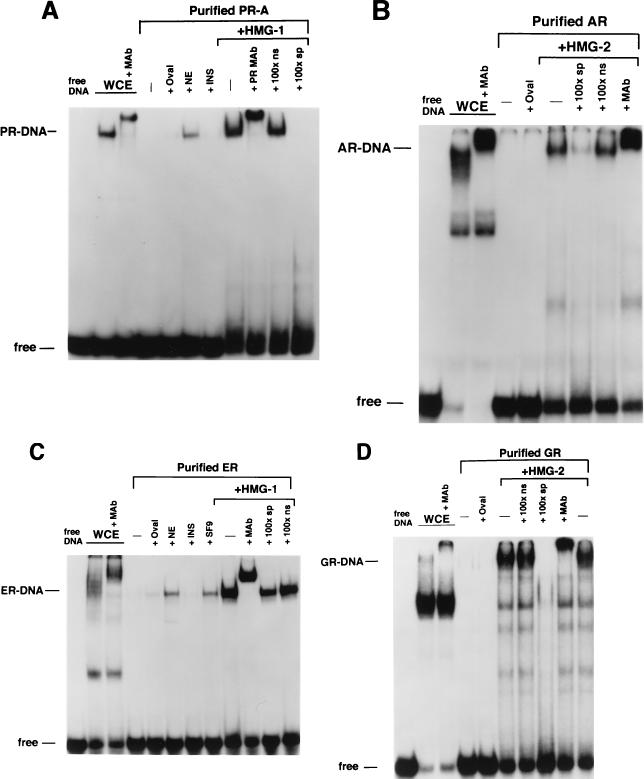
FIG. 4.
HMG-1/-2 stimulate the sequence-specific DNA binding activity of all steroid receptors tested. (A) Equal amounts (30 nM) of polyhistidine-tagged PR-A in Sf9 whole-cell extract (WCE) or affinity-purified PR-A were analyzed for binding to a 32P-labeled palindromic GRE-PRE oligonucleotide by EMSA. Lanes contain Sf9 whole-cell extract (WCE), purified PR alone (−), or purified PR plus 1 μg of ovalbumin (+Oval), 1 μg of nuclear extract (+NE) from calf thymus, 1 μg of insulin (+INS), or 300 ng of purified calf thymus HMG-1 (+HMG-1). The HMG-1-stimulated PR-DNA complexes were supershifted by the PR-specific MAb, AB-52, and were competed by a 100-fold molar excess of the PRE oligonucleotide (100×sp) but not by an ERE oligonucleotide (100×ns). (B) Equal amounts (70 nM) of baculovirus-expressed AR in WCE and affinity-purified AR were analyzed for binding to the GRE-PRE oligonucleotide by EMSA as described for panel A. (C) Equal amounts (10 nM) of baculovirus-expressed ER in WCE and affinity-purified ER were analyzed for binding to an ERE oligonucleotide by EMSA as described for panel A with or without the addition of 300 ng of purified calf thymus HMG-1. (D) Equal amounts (200 nM) of baculovirus-expressed GR in WCE and affinity-purified GR were analyzed for binding to the GRE-PRE by EMSA as described for panel A. All receptors were bound to their cognate (see Materials and Methods) hormonal ligands in Sf9 cells during expression.