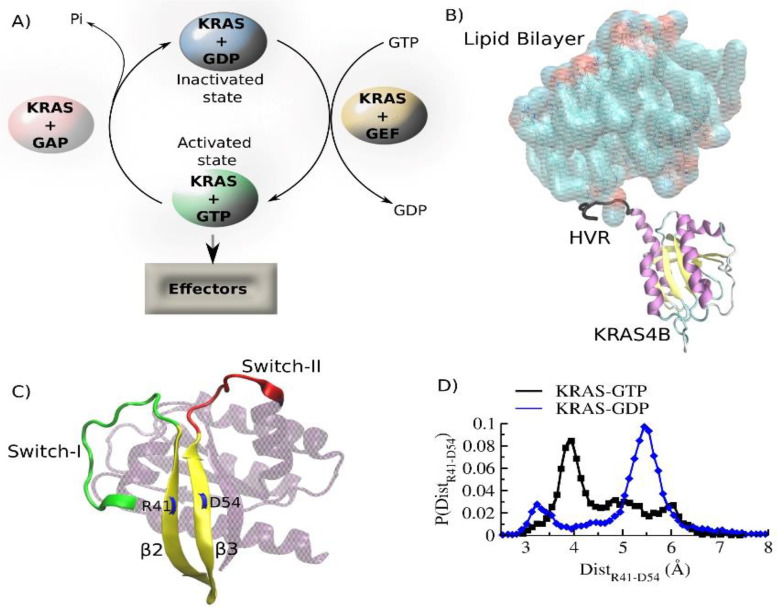
FIG. 6.
The structure/function cycle of KRAS. (a) Activation/deactivation cycle of KRAS GTPase. GDP/GTP exchange in this cycle is mediated by two other proteins: guanine nucleotide-exchange factors (GEFs) and GTPase activating proteins (GAPs). While GEFs catalyze the exchange from GDP to GTP, GAPs enhance the rate of exchange from GTP to GDP. (b) NMR-driven structure of KRAS4B-GTP on a lipid bilayer (pdb id:6W4E) (Lee et al., 2020). The positively charged intrinsically disordered hypervariable region (HVR) is shown in gray to highlight its mode of association with the lipid membrane. (c) Three-dimensional structure of inactive GDP-bound human KRAS highlighting the dynamic switch regions: Switch I (green) and Switch II (red) (pdb id: 4OBE) (Hunter et al., 2014). These two switch regions are connected via two parallel β strands: β2 and β3. (d) The switch dynamics and their correlation with β2-β3 fluctuation are compared in GDP and GTP-bound states. The dynamics are assessed by quantifying the distance between two residues R41 (located in β2) and D54 (located in β3). The distance distribution indicates enhanced conformation fluctuation of the switches in the GDP-bound state. [Adapted from Vatansever et al., Sci. Rep. 6, 37012 (2016). Copyright 2016 Author(s), licensed under a Creative Commons Attribution 4.0 License.]