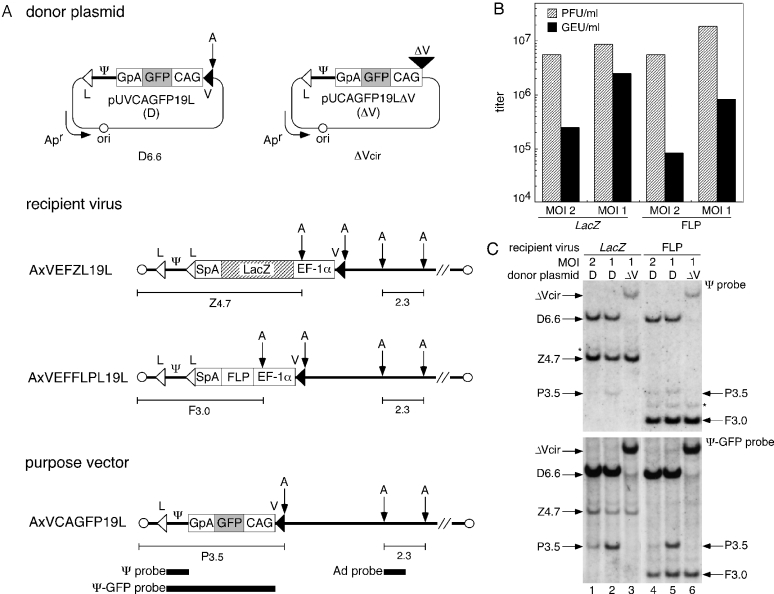
Figure 2.
Production of a first-generation adenovirus vector by Cre-mediated RMCE. (A) Structure of the donor plasmids, recipient viruses and purpose vector. pUVCAGFP19L (top left), donor plasmid carrying a GFP-expression unit flanked by L and V. pUCAGFP19LΔV (top right), control plasmid lacking V. AxVEFZL19L and AxVEFFLPL19L, LacZ and FLP recipient viruses, respectively. AxVCAGFP19L, purpose vector. The position of the probes (closed bars, bottom) and the size (kb) of the specific bands corresponding to fragments from the recipient viruses (Z4.7 or F3.0), the purpose vector (P3.5) and the adenovirus constant region (2.3) detected by Southern blotting are shown. V, a mutant loxP V; CAG, CAG promoter; GFP, green fluorescent protein; GpA, rabbit β-globin poly(A) signal; Ψ, viral-packaging signal; L, wild-type loxP; Apr, ampicillin-resistance gene; ori, plasmid replication origin; EF-1α, EF-1α promoter; SpA, SV40 early poly(A) signal; and A, AflII site. (B) Virus titer of the produced purpose vector and residual recipient viruses in passage 1 stock. The indicated MOIs are those for the initial infection of the 293FNCre cells with the recipient viruses. PFU, plaque-forming units; GEU, GFP-expressing units. (C) Detection of the infectious viral genomes in passage 1 stock. 293FNCre cells were infected with LacZ (lanes 1–3) or FLP (lanes 4–6) recipient viruses at an MOI of 2 or 1 and cultured in a 6-well plate, followed by transfection with the donor plasmid (D) or the control plasmid (ΔV) at 3.0 μg/well. The passage 1 stock was prepared on day 5 and then used to infect CV1 cells to detect the infectious viral genomes. Specific bands corresponding to fragments from the purpose vector (P3.5) and each recipient virus (LacZ, Z4.7; FLP, F3.0) were detected using Southern blotting with Ψ (upper panel) or Ψ-GFP probes (lower panel) using the same nylon membrane. The bands representing D6.6 and ΔVcir, corresponding to AflII-cleaved 6.6 kb pUVCAGFP19L donor and circular pUCAGFP19LΔV, respectively, appear to be ‘carryover’ DNA initially transfected to the 293FNCre cells and then co-transferred to the CV1 cells, together with the adenoviral infection (36). These bands were not detected at and after the second cycle of infection (data not shown). Note that the DNA was detected as an AflII-digested linear form of the donor plasmid and a circular form of the control plasmid. The bands indicated by the asterisk (*) appear to have been caused by recipient viruses carrying two packaging signals generated by the re-integration of the excised circular Ψ into either loxP site on the recipient viral genomes.