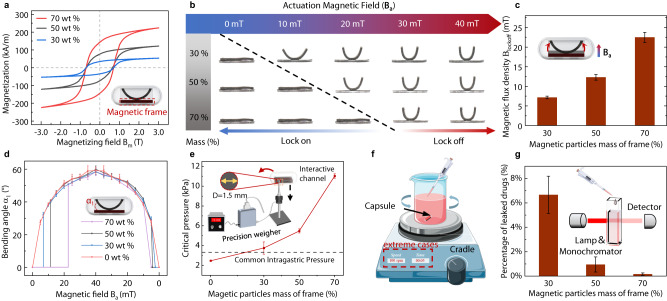
Fig. 2. Magnetic characterization and sealing test of MagCaps.
a Magnetic hysteresis loops of the magnetic soft composites containing NdFeB microparticles and PDMS. Source data are provided as a Source Data file. b Deformation profiles of the magnetic leaf when the applied magnetic field strength increased from 0 mT to 40 mT. c Magnetic fields required to open the magnetic valve for magnetic frames with the magnetic powder content are set to 30%, 50%, and 70% (n = 3, data are presented as mean values +/− SD). d Measured bending angles with different magnetic powder content under the static magnetic field (n = 3, data are presented as mean values +/− SD). e Critical pressures required for the magnetic leaf detachment from the magnetic frame with varying magnetic powder mass fractions (n = 3, data are presented as mean values +/− SD). f Schematic diagram of the sealing test, where the capsules containing the drug were placed on a shaker and tested at the extreme case. g Sealing performance of capsules for magnetic frames with different magnetic powder content, which can be revealed by the percentage of leaked drugs (n = 3, data are presented as mean values +/− SD). Source data are provided as a Source Data file. The Fig. 2e–g were partly generated using Servier Medical Art, provided by Servier, licensed under a Creative Commons Attribution 3.0 unported license71.