Abstract
Peroxisome proliferator-activated receptor gamma coactivator-1 (PGC-1) family (PGC-1s), consisting of three members encompassing PGC-1α, PGC-1β, and PGC-1-related coactivator (PRC), was discovered more than a quarter-century ago. PGC-1s are essential coordinators of many vital cellular events, including mitochondrial functions, oxidative stress, endoplasmic reticulum homeostasis, and inflammation. Accumulating evidence has shown that PGC-1s are implicated in many diseases, such as cancers, cardiac diseases and cardiovascular diseases, neurological disorders, kidney diseases, motor system diseases, and metabolic disorders. Examining the upstream modulators and co-activated partners of PGC-1s and identifying critical biological events modulated by downstream effectors of PGC-1s contribute to the presentation of the elaborate network of PGC-1s. Furthermore, discussing the correlation between PGC-1s and diseases as well as summarizing the therapy targeting PGC-1s helps make individualized and precise intervention methods. In this review, we summarize basic knowledge regarding the PGC-1s family as well as the molecular regulatory network, discuss the physio-pathological roles of PGC-1s in human diseases, review the application of PGC-1s, including the diagnostic and prognostic value of PGC-1s and several therapies in pre-clinical studies, and suggest several directions for future investigations. This review presents the immense potential of targeting PGC-1s in the treatment of diseases and hopefully facilitates the promotion of PGC-1s as new therapeutic targets.
Subject terms: Pathogenesis, Diseases
Introduction
Peroxisome proliferator-activated receptor (PPAR) gamma coactivator-1 (PGC-1) family (PGC-1s) consist of three members, namely PGC-1α, PGC-1β, and PGC-1-related coactivator (PRC). The first member to be discovered was PGC-1α, which plays important roles in modulating mitochondrial functions in brown adipose tissue (BAT) and skeletal muscle.1 The amino acid sequence of these three members shares considerable homology in both the N- and C-terminal ends of the proteins, which partially explains their similar features and functionalities. Originally, PGC-1s were acknowledged as pivotal regulators in mitochondrial function and energy metabolism. They exert significant roles in mediating oxidative phosphorylation (OXPHOS), fatty acid/lipid metabolism, and reactive oxygen species (ROS) detoxication.2–4 Considering their intrinsic capacity to coordinate cellular bioenergetics, it is not surprising that PGC-1s have diverse functions in a diverse array of diseases, such as but not limited to cancers, cardiovascular diseases, and neurological disorders. PGC-1s achieve these by activating coactivated genes such as estrogen-related receptors (ERRs), PPARs, and nuclear respiratory factors (NRFs).5–9 Importantly, with the development of research in the past two decades, increasing evidence supported the potential application of targeting PGC-1s therapies.10–12
In this paper, our aim is to provide a systematic and comprehensive summary of the architecture, upstream signals and parallel partners, biological function, and relation to health and diseases of PGC-1s. Furthermore, we also provide insights into the therapy targeting PGC-1s and suggest directions for future investigations. The compilation of information in this paper serves as a comprehensive repository, with the hope of illuminating the possibility of PGC-1s as novel therapeutic targets in the future.
Introduction and function Of Pgc-1s
The discovery history of PGC-1s
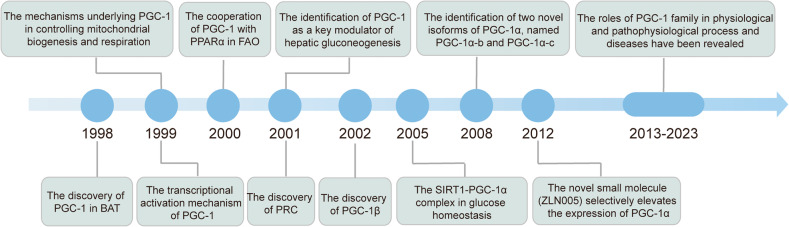
The history of PGC-1s can be traced back to its founding member, PGC-1α, which was identified in 1998 as a transcriptional coactivator of PPARγ in BAT, where it drives adaptive thermogenesis1 (Fig. 1). Subsequent studies revealed that the docking of PGC-1 to PPARγ stimulates a conformational change in PGC-1, which permits binding of SRC-1 and CBP/p300, thus resulting in increased transcriptional activity.13 In addition, Wu et al. elucidated the mechanisms by which PGC-1 controls mitochondrial biogenesis and respiration.2 PGC-1 was also recognized as a key modulator in fatty acid oxidation (FAO) and hepatic gluconeogenesis14,15 (Fig. 1). Two other members of PGC-1s family, PGC-1β and PRC, were discovered through sequence homology searches16,17 (Fig. 1). In 2008, the two novel isoforms of PGC-1α, PGC-1α-b and PGC-1α-c, were first identified. These isoforms are shorter than PGC-1α by 4 and 13 amino acids, respectively, and are transcribed by a novel exon located 13.7 kb upstream to the previously reported exon of the PGC-1α gene.18 In this text, unless the variant is specifically specified, “PGC-1α” refers to the original PGC-1α gene/protein. In 2012, Zhang et al. discovered a novel small molecule, known as ZLN005, which selectively elevates the expression of PGC-1α.19 However, despite extensive studies on the association between PGC-1s and various physiological and pathophysiological process and diseases, no drugs targeting PGC-1s have achieved the application from bench to bedside. Therefore, a more comprehensive understanding of PGC-1s is necessary to improve PGC-1s-related therapies for the precise intervention and management of different diseases.
Fig. 1.
A brief history of the PGC-1s family. The figure describes the milestones of PGC-1s from the origin of different members to the most advanced scientific discoveries, including the identification of biological functions, development of activator, and recent progresses in human health and diseases
The structure of PGC-1s
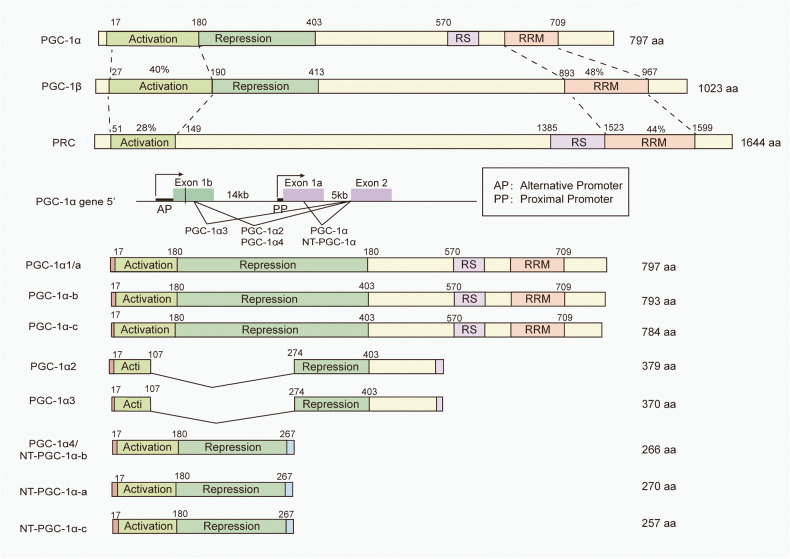
PGC-1α and PGC-1β have the highest sequence identity, particularly in several domains including the N-terminal activation domain (40% homology) and the C-terminal RNA binding domain (48% homology).20,21 They are both predominantly expressed in tissues that demand high energy consumption, such as BAT, heart, and brain.1,16 PRC is expressed in all tissues and shares lower levels of homology compared to the other two members.17 It remains poorly characterized and known, greatly because of the embryonic lethal phenotype of PRC knockout mice.22
The N- and C-terminal ends of the three members are highly homologous (Fig. 2). The N-terminal region of PGC-1s contains conserved leucine-rich LXXLL motifs and acts as activation domain. This domain is responsible for recruiting histone acetyltransferase proteins, including steroid receptor coactivator (SRC)-1 and cAMP response element-binding (CREB) binding protein/p300.13 These histone acetyltransferase proteins facilitate the remodeling of histones within chromatin and further increase the transcriptional activity of PGC-1s. Adjacent to the N-terminal region of PGC-1α/β is a domain that represses their own activity, known as the repression domain (RD). The C-terminal region encompasses a well-conserved RNA recognition motif (RRM), which participates in RNA alternative splicing.23 Moreover, the N-terminal of RRM, known as serine/arginine-rich stretch domain, also plays an important role in mRNA splicing. This is unique to PGC-1α and PRC, not found in PGC-1β.16,24 Host cell factor (HCF) acts as a coactivator to regulate gene expression during cell cycle progression and enhances the transcriptional activity of PGC-1s.16 In addition, the C-terminal region of PGC-1s contains several binding sites for other transcription factors, including forkhead box O (FOXO) 1 and yin yang 1 (YY1).25,26 PGC-1s have been demonstrated to co-activate transcription factors, such as PPARs, NRFs, and ERRs, which regulate the expressions of genes implicated in mitochondrial biogenesis, oxidative stress, and energy metabolism.27–30 Consequently, PGC-1s are recognized as one of the principal regulators in diverse cellular events.
Fig. 2.
Domain structure of the PGC-1s family and PGC-1α isoforms. The N-terminal region of PGC-1s is a conserved activation domain (AD). Adjacent to the N-terminal region of PGC-1α/β is a domain that represses their own activity, called the RD. The C-terminal region encompasses a well-conserved RRM, which participates in RNA alternative splicing. Moreover, the N-terminal of RRM also plays an important role in mRNA splicing, known as RS domain, which only exists in PGC-1α and PRC, but not in PGC-1β. Moreover, the existence of several promoter regions of a single PGC-1α, along with alternative splicing, leading to the production of PGC-1α isoforms. PGC-1α (also named PGC-1α1or PGC-1α-a) and NT-PGC-1α-a are transcribed by the proximal promoter of PGC-1α gene. Other PGC-1α isoforms are transcribed by a novel exon 1, located 13.7 kb upstream to of the proximal transcription start site
Upstream modulators of PGC-1s
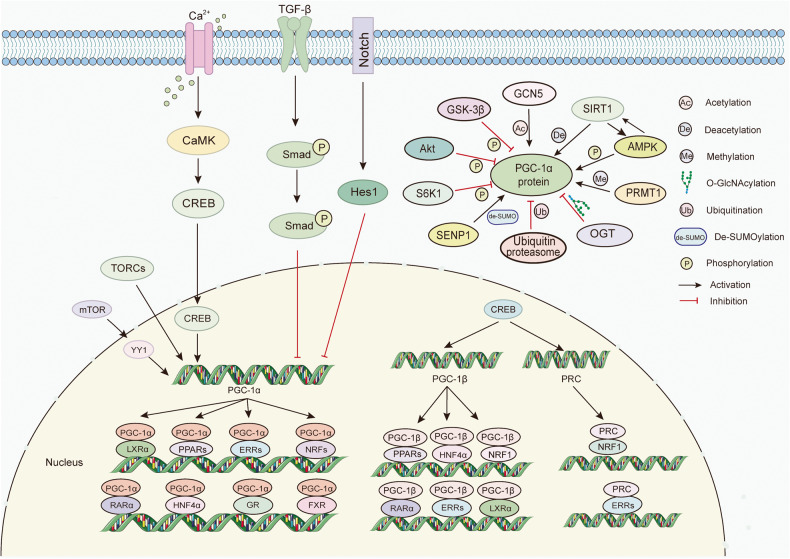
Numerous studies have reported that the expression of PGC-1s is extensively regulated by transcriptional and post-translational alterations in response to various external stimuli. For example, exercise enhances a pronounced anti-inflammatory phenotype that visceral adipose tissue possesses during aging, which is linked to the upregulated mRNA levels of PGC-1α.31 Protein post-translational modifications, including phosphorylation,32 deacetylation,33 and methylation,34 further broaden the dimensions of the regulatory network and play critical roles in the translocation and activation of PGC-1s. We will concentrate on a couple of upstream modulators, which exert indispensable roles in these modifications, providing a comprehensive and detailed landscape for the regulation of PGC-1s (Fig. 3).
Fig. 3.
The transcriptional regulatory mechanism and coactivators of the PGC-1s. Upstream modulators, such as YY1, CREB, Smad, Hes1, and TORCs regulate the transcriptional activity and levels. Moreover, PGC-1s play indispensable roles in various cellular events by coactivating transcription factors, including PPARs, ERRs, NRFs, HNFs, LXR, FXR, RARα, and GR. The expressions of PGC-1α are extensively regulated by post-translational alterations. For example, AMPK promotes the activity of PGC-1α by phosphorylation, while Akt, GSK-3β, and S6K1 inhibits PGC-1α by phosphorylation. GCN5 and SIRT1 mediates the deacetylation and acetylation of PGC-1α, respectively. Moreover, GlcNAc transferase (OGT) O-GlcNAcylate PGC-1α, thus protecting it from degradation protein arginine, while PGC-1α can be rapidly degraded in the nucleus through the ubiquitin-proteasome system. PGC-1α is inhibited by SUMOylation, and SENP1 facilitates the activity of PGC-1α through de-SUMOylation. Protein arginine methyl-transferase 1 (PRMT1) methylates PGC-1α, contributing to the induction of endogenous target genes of PGC-1α. These post-translational modifications further broaden the dimensions of the regulatory network and perform critical roles in PGC-1α translocation and activation
CREB and TORC
The transcriptional regulation of PGC-1α is orchestrated predominantly by the critical transcriptional factor CREB activation because the PGC-1α gene possesses a well-conserved binding site for CREB. In muscle cells, calcium-signaling components modulate the expression of PGC-1α, in which CREB is a key player. CaMKIV, as the calcium-dependent kinase, activates CREB, which in turn, binds to a conserved cAMP response element in the promoter of PGC-1s.35 Herzig et al. elucidated the activation mechanism of gluconeogenic genes during fasting.14 Specifically, during prolonged fasting, CREB potentiates gluconeogenic genes including phosphoenolpyruvate carboxykinase (PEPCK), pyruvate carboxylase, and glucose-6-phosphatase (G6P) by increasing the expression of PGC-1 in the liver.14 Moreover, during osteoclastogenesis, CREB directly targets PGC-1β, as it binds to the two CRE elements located 5.4 kb and 4.2 kb upstream in the PGC-1β promoter.36
Transducers of regulated CREB-binding proteins (TORCs) are generally considered to promote CREB-dependent gene transcription.37 Wu et al. screened 10,000 human full-length cDNAs and identified TORCs as upstream regulators of PGC-1α. When TORCs are forcefully expression in primary muscle cells, it induces its downstream target genes involved in the mitochondrial respiratory chain and TCA cycle, which largely depends on PGC-1α.38
In summary, CREB and TORCs strongly induce the PGC-1α signaling pathway, linking external signals to the transcriptional program of cellular events.
SIRT1 and GCN5
Silent information regulator sirtuin 1 (SIRT1) acts as a cellular sensor to detect energy availability and plays a variety of pivotal roles in cellular biology, such as inflammation, metabolism, oxidative stress, and apoptosis.39 As the first identified deacetylases for PGC-1α, SIRT1 requires the coenzyme NAD+ as a substrate for its function and is activated when the amounts of NAD+ or NADH or the NAD + /NADH ratio in cells change.40 Once activated, SIRT1 interacts with and deacetylates PGC-1α at specific lysine residues, in a NAD + -dependent manner, further promoting FAO and gluconeogenesis.33,40 In the liver, SIRT1 knockdown results in mild hypoglycemia, increased systemic glucose and insulin sensitivity, and decreased glucose generation. On the other hand, overexpression of SIRT1 reverses these changes, relying on the presence of PGC-1α.41 Notably, SIRT1 also plays a crucial role in regulating mitochondrial bio-oxidation synthesis in a PGC-1α dependent manner.42 PGC-1α and SIRT1 are localized in the mitochondrial matrix in the cytoplasm. The activation of PGC-1α by SIRT1-mediated deacetylation interacts with mitochondrial transcription factor A (TFAM), then enhancing TFAM coactivation and more efficient mitochondrial DNA (mtDNA) transcription. This is accompanied by the augmented activity of nuclear PGC-1α, allowing for the concomitant transcription of nuclear-encoded mitochondrial genes. This supports the idea that PGC-1α and SIRT1 are at the center stage of mitochondrial-nuclear communications.42
Considering that deacetylation is a reversible process, it is not surprising that PGC-1α can be mastered through acetylation. GCN5 has been identified to be the specific acetyltransferase for PGC-1α.43 GCN5 induces the translocation of PGC-1α to subnuclear domains and represses its function, ultimately repressing PGC-1α-induced gluconeogenic gene expressions and hepatic glucose secretion.43 SRC-3 knockout mice exhibit a more favorable metabolic profile compared to wild-type (WT) littermates, which is attributed to enhanced mitochondrial function and energy expenditure following PGC-1α activation. Specifically, SRC-3 enhances the expression of GCN5, thereby facilitating PGC-1α acetylation.44 Additionally, PGC-1β can be acetylated by GCN5 on at least 10 lysine residues located throughout the protein. Importantly, GCN5 greatly represses PGC-1β-induced endogenous target genes, including medium chain acyl CoA-dehydrogenase and glucose transporter 4 (GLUT4), further blunting the response to glucose transport induced by PGC-1β, illustrating that the acetylation of PGC-1β by GCN5 plays a crucial role in the modulation of glucose and lipid metabolism.45
Therefore, GCN5 and SIRT1 appear to function as a yin-yang pair, responsible for regulating the activity of PGC-1s. Conducting additional research on whether the activity of GCN5 and SIRT1 is also oppositely influenced by internal and external stimuli may contribute to the therapeutic applications of PGC-1s.
AMPK
AMP-activated protein kinase (AMPK), a member of the serine/threonine kinase group, serves as the metabolism guardian by participating in sensing the availability of nutrients and energy.46,47 When there are changes in energy availability and thus fluctuations in the adenosine triphosphate (ATP)/adenosine diphosphate (ADP) or ATP/adenosine monophosphate (AMP) ratio, AMPK is activated. As a result, activated AMPK restores energy homeostasis by promoting catabolic pathways and restraining anabolic pathways.48,49 Importantly, activated AMPK not only increases the transcription of PGC-1α,50,51 but also directly phosphorylates PGC-1α protein at threonine-177 and serine-538, ultimately ameliorating mitochondrial function, energy metabolism, and insulin resistance.32,52
Interestingly, AMPK modulates the deacetylation of PGC-1α by SIRT1, which explains many convergent biological effects of AMPK and SIRT1 on energy metabolism.53,54 During fasting and after exercise, AMPK serves as an initial sensor of energy stress to regulate nicotinamide phosphoribosyl transferase expressions and intracellular NAD+ levels, which in turn affects the activity of SIRT1 on downstream targets such as PGC-1α.54 The AMPK activator 5-aminoimidazole-4-carboxamide-1-b-D-riboside (AICAR) significantly increases PGC-1α activity on its own promoter in C2C12 myocytes, but this increment reduces over 60% in SIRT1-/- mouse embryonic fibroblasts. The absence of SIRT1 also compromises AICAR-induced PGC-1α-dependent transcriptional activity on other target genes.55
Akt
Protein kinase B (PKB, also known as Akt), a conserved serine/threonine kinase member of the AGC family of proteins, is considered to be expressed at the crossroads of multiple cellular processes.56 Interestingly, PGC-1α binds and coactivates FOXO1 in a manner that is inhibited by Akt-mediated phosphorylation, thus participating in insulin-regulated hepatic gluconeogenesis.25 Moreover, Akt has the ability to stabilize the Cdc-like kinase 2 (Clk2) protein, which phosphorylates the serine-arginine domain of PGC-1α and represses the activity of PGC-1α.57 Of note, there are three isoforms of Akt (Akt1, Akt2, and Akt3), which have overlapping and distinct roles and sometimes even perform contrasting functions.58 Several studies have explored the roles of Akt isoforms in regulating PGC-1α. Akt2 can directly phosphorylate PGC-1α at Ser 570, which further prevents the recruitment of PGC-1α to the cognate promoters, ultimately inhibiting gluconeogenesis and FAO.59 Akt2 ablation initially increases the mitochondrial volume and upregulates PGC-1α.60 Wright et al. showed that Akt3 silencing increases the cytoplasmic accumulation of PGC-1α, and reduces the expression of PGC-1α target genes.61 They further confirmed that Akt3 blockade increases chromosome maintenance region-1 (CRM-1, a major nuclear export receptor) expression to enhance PGC-1α nuclear export instead of direct effects on post-translational modifications of PGC-1α.62 However, Akt1 activation leads to an increment in the expression of PGC-1α, which increases mitochondrial biogenesis and induces apoptosis resistance, further contributing to the pathogenesis of pulmonary fibrosis.63 In brief, the different modulation of PGC-1α by Akt isoforms may be due to diverse regulatory levels and cellular processes, and more comprehensive investigation regarding the exact mechanism of Akt isoforms in regulating PGC-1α are required.
GSK-3β
Glycogen synthase kinase 3β (GSK-3β) is also a busy serine/threonine kinase, with over 100 known substrates to deal with.64 Among these substrates, one of the main targets is PGC-1α.65,66 Olson et al. discovered that PGC-1α contains two Cdc4 (the F-box component of the SCFCdc4 ubiquitin ligase) phosphodegrons that bind to Cdc4, which results in SCFCdc4-mediated ubiquitylation and proteasomal degradation of PGC-1α. This process requires GSK3β-dependent phosphorylation at the T295 site.65 Interestingly, GSK3β-dependent phosphorylation is also required for nuclear degradation of PGC-1α in response to stress. When exposed to hydrogen peroxide, activated GSK-3β phosphorylates PGC-1α, leading to intranuclear proteasomal degradation, which is also observed in mice both in the oxidative stress response and caloric restriction (CR).66
Additionally, in skeletal muscle cells, the inactivation of GSK-3β potently increases the abundance of PGC-1α and oxidative metabolism.67,68 Further investigation has confirmed that the inactivation of GSK-3β results in the dephosphorylation of transcription factor EB (TFEB), which then induces the translocation of the TFEB protein to the nuclear. This in turn elevates the activity of the PGC-1α promoter, leading to increased expression and protein abundance of PGC-1α.69 Omi is a serine protease present in the mitochondrial space. Under stressful conditions, Omi is released into the cytosol, where it promotes apoptosis through both caspase-dependent and -independent pathways.70 The loss of Omi protease activity gives rise to the degradation of PGC-1α, in which GSK-3β is an essential mediator.71 Overall, PGC-1α functions as the downstream effector of GSK-3β, enabling GSK-3β to exert an indispensable function in various cellular events.
Epigenetic modulatory mechanisms of PGC-1s
Some epigenetic regulations, such as DNA methylation and miRNA regulation, also play an important role in modulating PGC-1s. Wu et al. discovered a growth arrest and DNA damage-inducible β (Gadd45β)-dependent pathway that promotes hepatic glucose production. Mechanistic study revealed that Gadd45β, in conjunction with ten-eleven translocation 1 (TET1), promotes DNA demethylation of the PGC-1α promoter, thereby stimulating PGC-1α expression and promoting gluconeogenesis and hyperglycemia.72 In type 2 diabetes mellitus (T2DM) patients, the methylation levels of PGC-1α promoter in skeletal muscle, adipose tissue, and pancreatic islet cells are higher compared to normal individuals.73,74 Additionally, PPARGC1A methylated DNA/unmethylated DNA ratio in the liver has a significant correlation with plasma fasting insulin levels and homeostasis model assessment of insulin resistance.75 Interestingly, acute endurance exercise can induce the reposition of -1 nucleosome from the transcriptional start site and decreases the methylation level of -260 nucleotide, promoting the transcription of PGC-1α.76 These data suggest that DNA demethylation links PGC-1α with metabolic disturbance.
Moreover, several miRNAs have been confirmed to directly target PGC-1α, thus playing crucial roles in various biological processes.77–83 For example, the 3’-untranslated region (UTR) of PGC-1α mRNA revealed two conserved miR-23a sites. The activation of miR-23a inhibits gluconeogenesis in hepatocellular carcinoma by decreasing the level of G6P and PGC-1α.83 Du et al. found that the suppression of miR-23a restores the PGC-1α/p-dynamin-related protein 1 (Drp1) cascade, which improves mitochondrial membrane potential (MMP) and inhibits oxidative stress and cardiomyocyte apoptosis, thereby improving doxorubicin-induced cardiotoxicity.78 Moreover, miR‑696 also play an important role in gluconeogenesis and insulin resistance by downregulating PGC-1α.84 A luciferase reporter assay indicated the direct recognition of miR‑696 in a specific location within the 3’-UTR of PGC-1α transcripts.84 miR-696 overexpression also impedes mitochondria biogenesis and FAO by inhibiting PGC-1α.85 In the future, gaining a comprehensive understanding of miRNA regulation in PGC-1α provides hope for developing miRNA agents targeting PGC-1α.
Others
In addition to the main modulators, a diverse set of molecules or modification modes that can effectively regulate the expression and activity of PGC-1s have also been well described.
At the transcription level, Smad3 induced by TGF-β directly binds to the promoter of PGC-1α to decrease the levels of PGC-1α in 3T3-L1 cells, which links TGF-β activity to glucose tolerance and energy homeostasis.86 Moreover, HES1, a gene targeted by Notch, is strongly negatively correlated with PGC-1α in human kidney tubule samples. The ChIP assay confirmed direct binding of Hes1 to the promoter region of PGC-1α.87 In addition, the mammalian target of rapamycin (mTOR) mediates the interaction between PGC-1α and YY1, leading to an increase in PGC-1α promoter activity.26
At the post-translational level, S6 kinase 1 (S6K1) is an identified phosphorylation modulator of PGC-1α. Lustig et al. demonstrated that S6K1 phosphorylates PGC-1α on Ser 568 and Ser 572 within its arginine/serine-rich domain.88 Further research has revealed that S6K1-mediated phosphorylation represses the PGC-1α coactivation on hepatocyte nuclear factor (HNF) 4α, thereby significantly impairing the ability of PGC-1α to promote gluconeogenesis in vitro and in vivo.88 Besides, protein arginine methyl-transferase 1 (PRMT1) methylates PGC-1α, contributing to the induction of endogenous target genes of PGC-1α.34 Moreover, HCF C1 has the capacity to recruit O-GlcNAc transferase (OGT) to O-GlcNAcylate PGC-1α, thus protecting it from degradation and promoting gluconeogenesis.89 Rytinki et al. revealed the role of SUMOylation in the regulation of PGC-1α. They found that a lysine residue 183 located in the N-terminal activation domain of PGC-1α undergoes reversible SUMOylation.90 The SUMO-specific protease 1 (SENP1) facilitates PGC-1α, which is necessary for the expression of mitochondrial genes and subsequent mitochondrial biogenesis.91 As mentioned above, PGC-1α can be rapidly degraded in the nucleus through the ubiquitin-proteasome system.65,92 In addition, synoviolin (Syvn)1/Hrd1/Der3, an ER-resident E3 ubiquitin ligase, can trap PGC-1β in the perinuclear region and directly ubiquitinate it, thus impairing energy metabolism.93
Partners and downstream effectors of PGC-1s
As irreplaceable nodal regulators in a variety of physiological processes, PGC-1s coactivate the expression of many partners, as exemplified by PPARs, ERRs, NRFs, HNFs, liver X receptor (LXR), farnesoid X receptor (FXR), retinoic acid receptor α (RARα), and glucocorticoid receptor (GR).27,94–99 In this section, we will describe the intimate association between the first four transcription factors and PGC-1s, courtesy of the most intensive research, and others will be shown in the Fig. 3.
PPARs
Just like their name suggests, PGC-1s are PPARs-interacting proteins and they synergistically participate in the development of many diseases. PPARs, originally cloned in 1990, belong to the extended nuclear hormone receptor family and consist of three isotypes known as PPARα, PPARβ/δ, and PPARγ, and are mainly expressed in the kidney, liver, small intestine, and heart.100–103 PGC-1s have been demonstrated to directly cooperate with PPARs in controlling the transcription of nuclear genes that encode FAO enzymes.15 Li and colleagues provided insight into the structural and biochemical basis behind the binding selectivity of PPARγ to PGC-1.104 The initial LXXLL motif has the strongest affinity for binding to PPARγ. Specifically, the ligand-binding domain of PPAR is composed of 13 helices and four short strands that are folded into a three-layer helical sandwich and different helix forms a charge-clamp pocket, where the LXXLL motif of PGC-1 is docked.104
In many animal models, researchers have emphasized the importance of their synergistic effects. For example, patatin-like phospholipase domain containing protein 2 (an adipose triglyceride lipase, also referred to as Atgl) can generate essential mediators involved in the lipid ligands production for PPARs activation. Atgl deficiency downregulates the mRNA levels of PPARα and PPARδ, which results in the decreased expression of PGC-1α and PGC-1β, followed by the severe disruption of mitochondrial substrate oxidation and respiration in the heart, ultimately causing excessive lipid accumulation, cardiac insufficiency, and lethal cardiomyopathy.28 This is in accord with that PPARα is crucial for BAT thermogenesis via induction of PGC-1α during lipid catabolism.105,106 Treatment with GW501516, which activates PPARδ, robustly upregulates the mRNA levels of lipid metabolism genes, but this effect is completely abolished when both PGC-1α and PGC-1β are absent.107 Apart from the regulation in transcription level, PPARβ modulates PGC-1α in post-translational modification. PPARβ binds to PGC-1α and limits its ubiquitination, which protects PGC-1α from degradation and increases the levels of PGC-1α, thus playing principal roles in the adaptive increase of mitochondrial enzymes in skeletal muscle by exercise.108
Meanwhile, PGC-1α performs critical biological functions through a PPARs-dependent pathway. Overexpression of PGC-1α in human epithelial ovarian cancer (OC) cell line Ho-8910 induces apoptosis through the coordinated regulation of Bcl-2 and Bax expression, However, this effect is partially hindered by the PPARγ antagonist GW9662 and suppression of PPARγ.109 Additionally, downregulated PGC-1α levels increase the expression of β-secretase, a key enzyme involved in amyloid-β (Aβ) production. However, PGC-1α does not affect Aβ and β-APP cleaving enzyme (BACE1) levels in N2a cells transfected with PPARγ siRNA or in PPARγ knockout fibroblasts.110 Intriguingly, PPARβ/δ activator GW501516 can upregulate PPARα levels, PPARα-DNA binding activity, and PPARα-target genes involved in FAO, reflecting the magnification effect of PPARβ in the PGC-1α-PPARα signaling system.111 Briefly, the aforementioned results underscore the existence of feedback mechanisms and interaction patterns between PGC-1s and PPARs, which take part in a spectrum of cellular events.
ERRs
ERRs are orphan members of the nuclear receptor superfamily and consist of three subtypes including ERRα, ERRβ, and ERRγ.112 In 2002, Huss and colleagues completed the identification of ERRα as a PGC-1α interacting partner by using a yeast two-hybrid approach.113 They discovered that ERRα binds to PGC-1α through a Leu-rich motif at amino acids 209-213 and utilizes additional LXXLL-containing domains as accessory binding sites rather than the LXXLL motif at amino acid position 142-146 of PGC-1α, which is distinct from that of other nuclear receptors of PGC-1α.113 Soon afterward, another team successfully confirmed these findings and the two levels regarding the modulation of ERRα by PGC-1. In one aspect, PGC-1 upregulates the mRNA expressions of ERRα in the heart, kidney, and muscle. In another aspect, PGC-1 interacts physically with ERRα and enables it to activate transcription.114
As one of the best-known partners of PGC-1s, ERRs are required for various functions of PGC-1s. These include regulating FAO-related enzyme, osteocalcin gene expression, mitochondrial biogenesis, glucose oxidation, adaptive metabolism response, and insulin sensitivity.114–124 For instance, the forced expression of PGC-1α in C2C12 myotubes induces both mRNA and protein expressions of pyruvate dehydrogenase kinase 4 (PDK4, a negative regulator of glucose oxidation), which is achieved by binding to ERRs.118 Furthermore, PGC-1α potently induces vascular endothelial growth factor (VEGF) expression and promotes angiogenesis. These findings suggest that PGC-1α coactivates the conserved binding sites of ERRα in the promoter and in a cluster within the first intron of the VEGF gene.125 In mice with double deficiency of PGC-1α and PGC-1β, the expression of CDP-diacylglycerol synthase 1 (Cds1, an enzyme that catalyzes the proximal step in cardiolipin biosynthesis) decreases, resulting in phospholipid abnormality. Further experiments have demonstrated that PGC-1α regulates ERRs to activate the transcription of Cds1.121 Under normal conditions, overexpression of either PGC-1α or PGC-1β upregulates protein synthesis and myotube diameter in C2C12 myotubes, while the suppression of ERRα weakens this effect.126 ERRα is also required for PGC-1β to stimulate carnitine/acylcarnitine translocase in C2C12 cells.127 Consistently, Kamei et al. discovered that PGC-1β functions as ERR ligand 1 and activates ERRs. Transgenic mice overexpressing PGC-1β/ERR ligand 1 exhibit increased expression of the medium-chain acyl CoA dehydrogenase, elevated energy expenditure, and resistance to obesity induced by a high-fat diet (HFD) or genetic abnormality. These findings validate that PGC-1β, acting as a protein-ligand of ERR, contributes to the control of energy balance.128
In summary, the PGC-1s-ERRs signaling pathway takes part in various essential biological functions. Coincidentally, ERRα has the ability to directly modulate the transcriptional activity of the PPAR and ERRα-mediated activation of FAO enzyme genes relies on the presence of PPAR.117 Additionally, ERRγ is implicated in the initial phase of PGC-1α-induced ERRα expression.129 These findings reflect complicated modulatory networks existing in different subtypes of the same coactivators of PGC-1s as well as different coactivators of PGC-1s.
NRFs
NRFs, composed of NRF-1 and NRF-2, were originally designated as the core promoter binding element for cytochrome c oxidase subunit IV, whereafter it was found to associate with the expression of nuclear genes encoding subunits of the five respiratory complexes, thereby playing key roles in the maintenance of mtDNA and respiratory chain function.130–133 Strikingly, Vercauteren et al. revealed that neither PGC-1α nor PRC directly binds to NRF-2 but they exist together in a complex in vivo. This complex formation is mediated by HCF-1, and all three are related to NRF-2-dependent nuclear genes that control the expression of the mitochondrial transcription factors, such as TFB1M and TFB2M.134–136 Besides, PGC-1α is activated during exercise and promotes the development of an endurance phenotype through interactions with PPARα, NRF-1, and NRF-2.137
HNFs
HNFs, categorized into four families, namely HNF1α/β, FOXA1/2/3, HNF4α/γ, and ONECUT1/2, are responsible for regulating genes involved in lipid homeostasis.138 The connection between HNFs and PGC-1s is particularly evident in glucose metabolism, lipoprotein metabolism, and response to fasting.139–143 For example, PGC-1α stimulates key genes involved in gluconeogenesis, such as PEPCK and G6P, but this ability is lost when HNF4α is absent.139 Moreover, the overexpression of PGC-1α also increases the mRNA of apolipoproteins A-IV, C-II, and C-III through a highly conserved HNF4α response element to interact with HNF4α.144 These data emphasize the crucial role of the PGC-1α/HNF4α partnership in nutrient metabolism. PGC-1α also plays a significant role in modulating the binding ability of HNF4α in response to cytokine treatment.145 While cytokine treatment does not dramatically change the protein levels of HNF4α and PGC-1α, it does reduce the recruitment of PGC-1α to HNF4α-binding sites, in turn downregulating the likelihood of the HNF4α-PGC-1α complex binding to HNF4α-binding sites.145
The roles of PGC-1s in biological functions and physiological processes
The effect of PGC-1s in mitochondrial functions
Mitochondria, serving as organelles responsible for energy generation in OXPHOS, are crucial for the activity, function, and viability of eukaryotic cells.146 Indeed, mitochondrial dysfunction has become an initiator and propagator in many pathological processes due to its inability to provide the required energy for tissues with eminent energy demand, such as the heart, brain, and muscles.147–149 Multiple investigations have established PGC-1s as master mediators in modulating mitochondrial functions. Mitochondrial biogenesis is an extremely intricate process that responds to the energy demand triggered by developmental signals or environmental stressors and new mitochondria are generated from the ones already present.150 This process involves the replication of mtDNA, coordinated expression of mitochondrial and nuclear genes, and the import of nuclear-coded mitochondrial proteins into the organelle and turnover.151 When activated by the upstream regulators or stressors mentioned earlier, PGC-1α is transferred from the cytoplasm to the nucleus and enhances the expression of NRFs. Subsequently, NRFs promote the transcription and expression of TFAM, which further boosts the transcription and replication of mtDNA and protein synthesis, ultimately leading to the generation of new mitochondria.2,134,152 Conversely, PGC-1α mutation impairs the transcription of TFAM, resulting in dysfunctional mtDNA replication.153 Simultaneously, the activation of PGC-1α stimulates the transcription of mitochondrial genes involved in respiratory chain complexes.1,154
Complementary to the process of mitochondrial biogenesis, mitochondrial quality control is indispensable for maintaining mitochondrial performance and adaptation. The mitochondrial proteins mitofusin (Mfn) 1/2, optic atrophy 1 (Opa1), and Drp1 mediate the fusion of the outer mitochondrial membranes, the fusion of the inner mitochondrial membranes, and the fission of mitochondrial, respectively.155,156 Importantly, aside from its well-established roles in mitochondrial biogenesis, PGC-1α also performs important functions in the dynamic properties of mitochondria, including fusion, fission, and degradation, which often orchestrate not only energy metabolism but also complex cell events.157,158 PGC-1α directly induces the transcriptional activity of the Mfn2 promoter and acts synergistically with Mfn2. The loss of Mfn2 reduces the stimulatory effect of PGC-1α on MMP, indicating the presence of a regulatory pathway involving PGC-1α and Mfn2.159,160 Moreover, PGC-1α overexpression counteracts the decrement in the expression of Mfn1/2 and Opa1.161,162 In contrast, the expression of Mfn1/2 is markedly downregulated in the muscle of the PGC-1α/β deficient mice compared to the other groups, accompanied by mitochondrial morphologic abnormalities, structural derangements, and fusion/fission and biogenic defects.160,163–165 Exercise training has been shown to reverse the mitochondrial network fragmentation and improve submaximal ADP-stimulated respiration in a PGC-1α-dependent manner.165 Emerging evidence also indicated that PGC-1α directly regulates the expression of Drp1 by binding to its promoter.166,167 Remarkably, upregulation of PGC-1α simultaneously increases the expression of Mfn2 and Opa1 while inhibiting the expression of Drp1 and fission 1 (Fis1), thus maintaining the balance between mitochondrial fission and fusion.168
Mitophagy is an autophagic mechanism that mediates mitochondrial degradation by specifically targeting and eliminating damaged mitochondria.169 A variety of studies uncovered the role of PGC-1α in regulating mitophagy. Overexpression of PGC-1α increases lysosomal capacity and indicators of autophagy flux, such as TFEB, LC3B, Beclin, and LAMP1, to maintain mitochondrial homeostasis.170,171 Exercise can enhance mitophagy, but this effect is reduced in the absence of PGC-1α.172 Furthermore, NRF-1 binds to the classic consensus site in the promoter of Fundc1 (a mitophagy receptor), thus enhancing mitophagy through its interaction with LC3.173 The PTEN-induced kinase 1 (PINK1) and Parkin RBR E3 ubiquitin-protein ligase pathway is the most predominant ubiquitination-dependent mitophagy pathway.174 Importantly, there is mutual antagonism between the PINK1/Parkin pathway and PGC-1α. PINK1 affects mitochondrial biogenesis by inhibiting the protein expressions of PGC-1α and mtDNA copy number. In turn, PGC-1α represses the protein expressions of PINK1/Parkin and the levels of mitophagy.175
As for PGC-1β, it is induced by CREB during osteoclast differentiation, which facilitates mitochondrial biogenesis and increases iron demand.36 3T3-L1 adipocytes overexpressing PGC-1β manifest broader and more ordered mitochondrial cristae, in parallel with elevated mtDNA, Fis1 mRNA expression, and intracellular ATP levels.176 In contrast, electron chain capacity, ATP synthesis, and OXPHOS are reduced in PGC-1β knockout mice.177–179 Meanwhile, the transcript levels of genes involved in mitochondrial protein import, such as Tomm40l, Timm44, and Timm8a1, and the transcript levels of Mfn2, Opa1, Drp1, and Fis1 are decreased in PGC-1β selectively ablated skeletal myofibers.180 These results suggested that PGC-1β is required for normal OXPHOS and mitochondrial function.
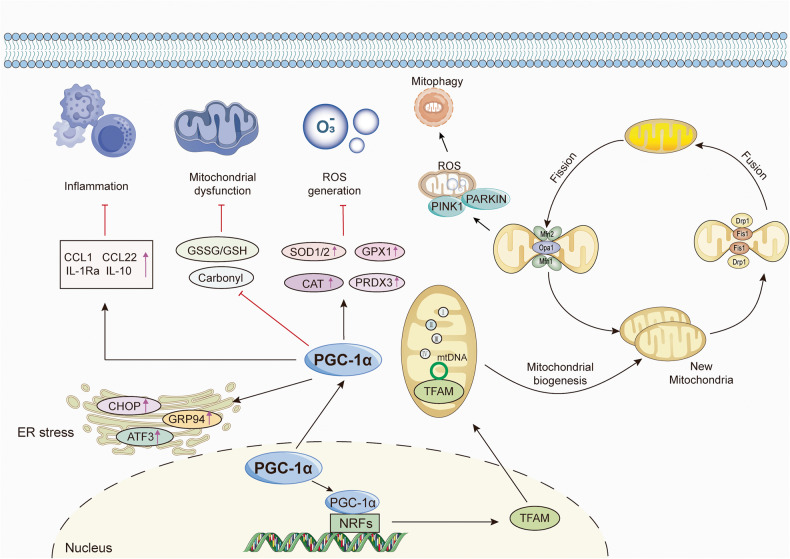
Taken together, as irreplaceable nodal regulators in mitochondrial activities, PGC-1α and PGC-1β participate in many vital mitochondrial biological events and establish a multi-link regulatory network based on the control of mitochondrial quality and quantity by regulating downstream effectors (Fig. 4).
Fig. 4.
Schematic representation of the critical regulatory roles of PGC-1s in biological functions. PGC-1s, especially PGC-1α, orchestrate the whole processes of mitochondrial life cycle, including mitochondrial biogenesis, fission, fusion, and mitophagy by modulating the coactivators and downstream effectors such as NRFs, Mfn1/2, Opa1, Drp1, and Parkin. PGC-1α fight against oxidative damage by upregulating a wide array of gene expressions regarding anti-oxidant proteins, including SOD, GPX, CAT, and PRDX3. Additionally, PGC-1α and PGC-1β play anti-inflammatory effects by inhibiting the pro-inflammatory factors. Moreover, PGC-1α can improve ER stress by upregulating CHOP, ATF3, and GRP94
The effect of PGC-1s in oxidative stress
Oxidative stress refers to an imbalance between the oxidant system and antioxidant defenses caused by the excessive production of ROS or reactive nitrogen species, terminally resulting in damage to DNA, proteins, and cell.181 Indeed, PGC-1s also fight against oxidative damage by upregulating a wide array of gene expressions associated with anti-oxidant proteins in different cells, tissues, and organs, including neurons, endothelial cells, retinal pigment epithelium (RPE), and liver.182–188
Under metabolic stress, PGC-1α interacts with and coactivates ERG, a fusion oncogene. The PGC-1α-ERG complex then drives the expression of antioxidant genes, including superoxide dismutase (SOD) 1 and thioredoxin (TXN), thus blunting ROS-mediated apoptosis.188 PGC-1α-/- retinas exhibit constitutive activation of the VEGF-A signaling pathway, which is partially reversed by antioxidant administration, suggesting that PGC-1α plays a significant role in angiogenesis by regulating ROS homeostasis.189 During the maturation of RPE, PGC-1α increases the expression of antioxidant genes, including catalase (CAT), glutathione peroxidase (GPX)1, peroxiredoxin (PRDX) 3, SOD1, SOD2, and TXN2, and represses oxidant-mediated cell death in RPE.184 Surprisingly, overexpression of PGC-1α even further inhibits the expression of PGC-1β in RPE. As an example of the transcriptional repression of PGC-1β by PGC-1α, the underlying molecular mechanism is unclear.184 In liver steatosis, PGC-1α expression is downregulated. Although hypoxia leads to a remarkable reduction in the expression of antioxidant genes in both PGC-1α+/+ and PGC-1α-/- hepatocytes, the restoration of antioxidant protein induced by re-oxygenation is generally diminished in PGC-1-/- hepatocytes, indicating that PGC-1α activity is particularly important in maintaining antioxidant gene expression following organ reperfusion.190 Even the loss of a single PGC-1α allele exacerbates oxidative stress and hepatic cell death, as shown by the elevated GSSG/GSH ratio and carbonyl content, further diminishing the murine host response to S. aureus peritonitis.191
Besides, FOXO3a directly regulates many genes that combat oxidative stress in vascular endothelial cells. Importantly, PGC-1α is required for this activity of FOXO3a, as PGC-1α deficiency severely curtails the expression of FOXO3a in endothelial cells.186 Friedreich’s ataxia is an autosomal recessive inherited disorder. Marmolino et al. found that PGC-1α and SOD-2 levels are decreased in FRDA cells but do not alter after the addition of hydrogen peroxide. However, PGC-1α siRNA causes a loss of SOD2 response to oxidative stress.192 Briefly, these studies revealed that PGC-1s are powerful regulators of ROS metabolism and anti-oxidant enzymes (Fig. 4).
The effect of PGC-1s in inflammation
Inflammation is an indispensable process that protects against adverse environmental factors by enforcing the defense of homeostasis and the functional and structural integrity of tissues and organs. However, persistent inflammation is regarded as a prime suspect in almost all diseases and underlies a wide range of physiological and pathological processes.193 PGC-1α is downregulated by various inflammatory mediators and cytokines.194,195 For example, tumor necrosis factor-α (TNF-α) reduces the expression of PGC-1α in the heart through nuclear factor-κB (NF-κB) and p38 mitogen-activated protein kinases (MAPK), leading to a notable enhancement in glucose oxidation rate.194,196 Likewise, TNF and interleukin (IL) 1 decrease PGC-1α and PGC-1β, as well as RXR, PPARα, PPARγ, and LXRα, in the liver cells.197,198 The similar phenomena also occur in proximal tubule cells, adipocytes, endothelial cells, and oligodendrocytes.199–202 Interestingly, NF-κB is constitutively bound to PGC-1α in human cardiac cells, which is further enhanced by TNF-α exposure, eventually giving rise to subsequent dysregulation of glucose oxidation.203
Importantly, the activation and upregulation of PGC-1α through genetic or pharmacological manipulation counteract inflammation and play protective roles in different pathological models.204–207 NOD-like receptor family-pyrin domain-containing 3 (NLRP3) is an essential sensor in the innate immune system and induces inflammation by promoting the release of the pro-inflammatory cytokines IL-1β and IL-18.208 PGC-1α has the ability to restrain the release of mtDNA from the mitochondria into the cytosol, oxidative stress, and increase TNFAIP3 (a negative regulator of NLRP3) to suppress NLRP3 inflammasome.209 In addition, both PGC-1α and PGC-1β inhibit p65 phosphorylation and PGC-1β blunts the transcription of p65 and p50 in the basal state, thus constraining inflammatory events in muscle cells.210,211 Moreover, PGC-1-dependent alteration of the cytokine profile is observed, featured by an upregulation in the anti-inflammatory factors, including CC chemokine ligand (CCL) 1, CCL22, IL-1Ra, transforming growth factor (TGF-β), and IL-10, and a remarkable inhibition of the pro-inflammatory factor IL-12.211 Based on these current studies, PGC-1s contribute to the anti-inflammatory environment in muscle and are important suppressors of inflammation (Fig. 4).
The effect of PGC-1s in endoplasmic reticulum homeostasis
The endoplasmic reticulum (ER), a complex and dynamic organelle, is responsible for the folding and trafficking of proteins that enter the secretory pathway. When ER functions are dysregulated and overwhelmed, the ER enters a stress state and the highly conserved unfolded protein response (UPR) are activated to restore ER homeostasis.212,213 Of note, there are reciprocal regulatory roles between PGC-1α and ER stress (Fig. 4). When faced with ER stressors, the mRNA levels of PGC-1α are markedly upregulated.214 Importantly, PGC-1α overexpression induces the expression of chaperones, such as BiP and GRP94, and the stress markers like ATF3 and CHOP. However, muscle-specific PGC-1α knockout mice show defective upregulation of ER chaperones and experience exacerbated ER stress after repeated exercise challenges. Mechanistic study has shown that PGC-1α plays an important role in the modulation of the UPR through coactivating ATF6α, a well-characterized sensor in UPR, thus contributing to skeletal muscle adapt to exercise training.214 Subsequently, Misra et al. illustrated that ERRγ binds to a responsive element in the ATF6α promoter, which requires the presence of PGC-1α.215 In acute kidney injury, overexpression of PGC-1α inhibits ER stress through the UPR pathway, thereby suppressing apoptosis via both the mitochondrial and ER pathways.216 Of interest, ER stress can in turn inhibit PGC-1α through suppressing C/EBPβ transcriptional activity, leading to mitochondrial dysfunction and subsequent diabetic embryopathy.217 Montori‑Grau et al. also observed that ER stress decreases PGC-1α expression in human myotubes and mouse skeletal muscle.218 Therefore, conducting more extensive investigation on PGC-1α and ER may provide novel insights into communications between mitochondria and ER.
The effect of PGC-1s in metabolism
Glucose metabolism refers to a series of complex chemical reactions, including glycolysis, aerobic oxidation, glycogen synthesis, and gluconeogenesis, which are necessary to meet the energy requirements of the vital organs.219 The roles of PGC-1s in glucose metabolism have been established, particularly in regulating gluconeogenesis and glucose uptake. In response to fasting, the increased synthesis and release of glucagon by pancreatic α cells binds to its receptor on hepatocytes and subsequently triggers the conformational change of G protein. Then, ATP is catalyzed to cAMP, which further binds to each regulatory subunit of protein kinase A (PKA), resulting in the translocation of PKA into the nucleus, finally phosphorylating CREB. The phosphorylated CREB upregulates the expression of PGC-1α. When PGC-1α is activated by CREB and TORCs or coactivates with HNF4α, PEPCK and G6P are increased, and hepatic glucose output is enhanced.144,220–222 After food intake, pancreatic β cells synthesize and release insulin that mediates the phosphorylation of Akt, which further triggers the phosphorylation of PGC-1α. The suppression of PGC-1α mediated by Akt results in impaired glucose homeostasis.59 PGC-1α also plays an inhibitory role in hepatic insulin resistance in animal models, such as HFD and Ob/Ob mice.223–225 Skeletal muscle is a primary site for the utilization of glucose. In skeletal muscle, the electro-transfection or overexpression of PGC-1α upregulates GLUT4 expression and glucose uptake.226,227 In addition, PGC-1α also increases FAO and glycogen synthesis and decreases glycolysis and glucose oxidation, thus upregulating muscle glycogen storage.228,229 Therefore, PGC-1α overexpression is harmful in the liver, where it facilitates hepatic glucose production. Conversely, it contributes to the oxidation and decrement of glucose in skeletal muscle. Of note, the roles of PGC-1β in glucose metabolism are not consistent with those of PGC-1α. The capacity of PGC-1β to stimulate gluconeogenic genes is relatively low, partially owing to its inability to coactivate with HNF4α and FOXO1.230 Nagai et al. confirmed that PGC-1β knockdown reverses hepatic insulin resistance caused by fructose in both basal and insulin-stimulated states.231 Therefore, deeper research focusing on the underlying mechanisms regarding the distinct roles between PGC-1α and PGC-1β may provide new insights for the treatment of abnormal glucose metabolism-related diseases.
Another noteworthy effect of PGC-1s is their roles in modulating lipid metabolism. For example, when PGC-1α is overexpressed in murine primary hepatocytes, triglyceride secretion is reduced and FAO is increased to meet energy needs during fasting.95 In accordance with this, Huang et al. discovered that PGC-1α stimulates peroxisomal activity and elevates long-chain and very-long-chain FAO in human primary myotubes.232 Interestingly, PGC-1α enhances lipogenesis in skeletal muscle.229,233 Mechanically, PGC-1α induces and coactivates LXR on the proximal promoter of fatty acid synthase, directly facilitating de novo lipid biosynthesis.233 PGC-1α also upregulates the mRNA and protein levels of FITM1/FIT1, which promotes the formation of lipid droplets.229 Besides, PGC-1α plays important roles in white adipose tissue browning and thermogenesis.234–236 Remarkably, gene expression array profiling revealed that PGC-1β, but not PGC-1α, induces the expression of several genes involved in converting glucose to fatty acid. This results from that PGC-1β interacts with carbohydrate response element binding protein (ChREBP) and binds to the liver-type pyruvate kinase promoter. This highlights the distinct and indispensable roles of PGC-1β in fatty acid synthesis (FAS).237 Nevertheless, when exposed to cold, PGC-1β knockout mice develop abnormal hypothermia and hepatic steatosis induced by HFD. Even the compensatory increase in PGC-1α is insufficient to counteract these effects.238 In a mouse model with constitutive hepatic activation of PGC-1β, methionine choline-deficient diet-induced hepatic steatosis is ameliorated, primarily relying on the ability of PGC-1β to drive FAO and citrate cycle, and induce triglyceride secretion.239 Liver-specific deletion of PGC-1β leads to impaired FAO capacity and mitochondrial dysfunction, giving rise to hepatic steatosis.240 The current data suggested that PGC-1β plays dual roles in governing hepatic fatty acid metabolism as it can regulate both FAO and FAS.
Besides, PGC-1α is implicated in amino acids metabolism. Overexpression of PGC-1α in the skeletal muscle increases the expression of enzymes related to branched-chain amino acid (BCAA) metabolism related, such as branched-chain aminotransferase (BCAT) 2 and branched-chain a-keto acid dehydrogenase (BCKDH), which promotes BCAA catabolism and downregulates the levels of BCAA, including valine, leucine, and isoleucine.241 Similarly, overexpression of PGC-1α increases BCAA genes and decreases valine levels, while muscle-specific PGC-1α knockout mice manifests downregulated expression of BCAA genes and levels of 3-hydroxyisobutyrate (a catabolic intermediate of valine).242,243 Further study has demonstrated that PGC-1α in myotubes stimulates the catabolism of valine to 3-HIB, which then enhances endothelial fatty acid uptake and promotes lipid accumulation in muscle, leading to insulin resistance in mice.243 Additionally, during fasting, PGC-1α enhances the promoter activity of alanine aminotransferase 2 (ALT2) in muscle cells in a dose-dependent manner, which facilitates alanine synthesis and secretion.244 Patients with T2DM exhibit more aggravating impairments in BCAA catabolism after a glucose load.242 These findings may reflect that PGC-1α conducts a cross-regulatory link among the amino acid catabolism, fatty acid metabolism, and glucose levels.
Overall, in light of the pleiotropic effects of PGC-1s in metabolism, especially in glucose and lipid metabolism, which depends on a high degree of specificity in different tissues, decrypting their roles in metabolism guides an approach to design better pharmacological treatment to attenuate metabolic diseases.
The isoforms of PGC-1α
Among the three founding members of the family mentioned above, PGC-1α has garnered extensive attention since its discovery over 20 years ago. Notably, in addition to the original PGC-1α discussed previously, several studies revealed the existence of several promoter regions of a single PGC-1α, along with alternative splicing, subsequently leading to the production of PGC-1α variants (Fig. 2). While these isoforms share some similarities in structures and overlapping functions, they still have many distinct properties. This section will specifically examine the structural and functional characteristics of PGC-1α variants.
PGC-1α-b and PGC-1α-c
In 2008, two novel isoforms of PGC-1α mRNA, named PGC-1α-b and PGC-1α-c, were discovered. Both isoforms are transcribed by a novel exon 1 (exon 1b), located 13.7 kb upstream to the previously reported exon 1 (exon 1a) of the PGC-1α gene. PGC-1α-b and PGC-1α-c are shorter than PGC-1α by four and 13 amino acids, respectively, and differ only in the N-terminal region of the 797 amino acid long murine full-length protein. As for the differences between the PGC-1α-b and PGC-1α-c, they come from the alternative splicing occurring within exon 1b, in which the upstream-splicing site is used for PGC-1α-b, whereas the downstream-splicing site is used for PGC-1α-c.18
Importantly, both PGC-1α-b and PGC-1α-c are functional. Specifically, overexpressing either PGC-1α-b or PGC-1α-c increases the expression of genes involved in mitochondrial biosynthesis and FAO. β2-AR agonist injection, endurance exercise, or resistance exercise leads to an increment in PGC-1α-b and PGC-1α-c mRNA in skeletal muscles.18,245,246 Interestingly, while a single bout of restricted blood flow exercise increases both PGC-1α-a and PGC-1α-b transcripts, the upregulation in PGC-1α-b is more significant.247 A randomized controlled trial revealed that exercise rapidly upregulates the mRNA and protein levels of PGC-1α-b, with the elevated protein occurring before that of total PGC-1α protein, emphasizing PGC-1α-b as the most exercise-responsive PGC-1 isoform.248 Additionally, exercise-induced mRNA responses of PGC-1α isoforms (PGC-1α, PGC-1α-b, PGC-1α-c) are intensity dependent.249 Yoshioka et al. found that the alternative promoter of the human PGC-1α gene can be activated by CaMKIV and calcineurin A. CaMKIV can recruit CREB to a putative CRE located downstream of the E-box, thereby activating the PGC-1α-b promoter in cultured myoblasts.250 These findings suggest a potential molecular basis by which exercise increases isoform-specific PGC-1α mRNA. Evidence from mice overexpressing PGC-1α-b protein in skeletal muscle further supports the notion that increasing PGC-1α-b protein or function is a useful strategy for sedentary subjects to exercise efficiently. PGC-1α-b overexpression promotes mitochondrial biogenesis 4-fold, increases the expression of fatty acid transporters, enhances angiogenesis in skeletal muscle 1.4 to 2.7-fold, and promotes exercise capacity by 35% and peak oxygen uptake by 20%, highlighting the importance of the induction and activation of PGC-1α-b in the adaptation to exercise training.251
NT-PGC-1α
Zhang et al. reported a novel truncated form of PGC-1α (NT-PGC-1α) composed of 267 amino acids of PGC-1α and 3 additional amino acids from the splicing insert.252 It contains the N-terminal domain, which recruits SRC-1 and CREB-binding protein and has the ability to activate transcription and interact with nuclear receptors. However, it loses key domains related to nuclear localization, interaction with other transcription factors, and protein degradation.252 Because of the absence of these sequences, NT-PGC-1α is primarily located in the cytosol (90%) under normal conditions. The highest levels of NT-PGC-1α protein expression are observed in the brain, while the liver has the lowest expression, and its expression in BAT and kidney is similar and intermediate between the liver and brain.252 NT-PGC-1α can physically interact with both PPARα and PPARγ and even exhibit stronger dependence on ligands compared to PGC-1α.252 Similar to PGC-1α, NT-PGC-1α is highly inducible by fasting, cold exposure, and exercise. Additionally, NT-PGC-1α transcript expression in resting muscle accounts for about half of the total PGC-1α expression after acute moderate-intensity exercise.252,253
Notably, ectopic expression of NT-PGC-1α in C2C12 myotube cells upregulates myosin heavy chain and GLUT4, promotes the expression of mitochondrial genes (Cyc1, COX5B, and ATP5B), and increases citrate synthase activity.254 In addition, NT-PGC-1α interacts with HNF4α and enhances HNF4α-mediated gene transcription, thus inducing gluconeogenesis in primary hepatocytes.255 When NT-PGC-1α is selectively expressed in PGC-1α-/- brown adipocytes, nuclear DNA-encoded mitochondrial genes, including TFAM are significantly upregulated, which is even more remarkable than PGC-1α-/- brown adipocytes expressing PGC-1α.256 Subsequently, Chang et al. identified the complete repertoire of PGC-1α and NT-PGC-1α target genes in BAT by unbiased genomic approach. Like PGC-1α, NT-PGC-1α targets a broad spectrum of genes related to ubiquitin-dependent protein catabolism, ribonucleoprotein complex biosynthesis, phospholipid biosynthesis, angiogenesis, glycogen metabolism, and autophagy.257 Furthermore, NT-PGC-1α overexpression increases the mRNA expression of PPARα-associated genes and suppresses phenylephrine-induced reductions in carnitine palmitoyl transferase 2 (CPT2) and acyl-coenzyme A dehydrogenase-medium chain (Acadm) expression, thereby regulating fatty acid metabolism, increasing extracellular oxygen consumption, and decreasing lipid droplet accumulation in neonatal rat cardiomyocytes.258 In contrast, NT‑PGC‑1α deficiency decreases mitochondrial FAO in BAT.259 Strikingly, the same group confirmed that NT-PGC-1α deficiency ameliorates HFD-induced obesity by reducing food intake, increasing fecal fat excretion, and decreasing fatty acid uptake in the intestine, adipose tissue, and liver.260 Although these results seem contradictory, which may be due to the different regulation in a particular process of fatty acid metabolism by NT-PGC-1α in different tissues, all these highlighted the role of NT-PGC-1α in regulating whole-body lipid homeostasis.
NT-PGC-1α-b and NT-PGC-1α-c are produced during cold exposure through the alternative first exon together with alternative splicing between exons 6 and 7.261 Furthermore, they are highly induced by low-, medium-, and high-intensity exercise, AICAR, and clenbuterol.254
PGC-1α2, PGC-1α3, and PGC-1α4
Using a targeted PCR strategy, PGC-1α2, PGC-1α3, and PGC-1α4 were cloned.262 PGC-1α2 and PGC-1α3 have different first exons but share the same remaining exon/intron structure, resulting in a similar domain structure except for discrete N termini at position.262 After a series of splicing events common to both PGC-1α2 and PGC-1α3, exons 4-6 and 9-13 are eliminated and exon 8 are spliced to the 3’ UTR of the PGC-1α gene, ultimately producing a common stop codon for both transcripts. The resulting proteins, PGC-1α2 and PGC-1α3 (379 and 370 amino acids long, respectively), contain part of the activation domain and repression domain and completely lack all the C-terminal motifs of PGC-1α. PGC-1α4 (which is identical to NT-PGC-1α-b mentioned earlier) possesses the same alternative exon1 with PGC-1α2 and thus the same N terminus. Unlike PGC-1α2 and PGC-1α3, the mRNA of PGC-1α4 contains a 31 nucleotides insertion between exons 6 and 7, therefore producing a premature stop codon. It is predicted to encode 266 amino acids, a protein of 29.1 kDa.262 Comparing the gene sets regulated by each PGC-1α isoform, PGC-1α2 and PGC-1α3 form a distinct cluster from PGC-1α4, which shows higher similarities with the genes targeted by PGC-1α. This indicates that the transcriptional activity of the PGC-1α isoforms is dictated by the conservation of the N-terminal activation domain rather than the presence or absence of the RS/RRM motifs.263
The researchers also found that cold exposure induces the expression of all PGC-1α variants in BAT.262 However, when examining the genes changes driven by different PGC-1α variants, it was discovered that PGC-1α2 and 3 only affect a very small number of genes that overlap with PGC-1α. The expression of PGC-1α4 in myotubes did not affect the regulation of many classic PGC-1α targets, including mitochondrial OXPHOS genes. In contrast, it specifically induces insulin-like growth factor 1 and represses myostatin, thus regulating skeletal muscle size.262 In response to the inflammatory signal mediated by TNF-α, PGC-1α4 also has distinct roles compared to PGC-1α1. PGC-1α1 primarily affects genes involved in nutrient metabolism and mitochondrial biology, and decreases the expression of a wide range of inflammatory genes, but it does not prevent hepatocyte death, while PGC-1α4 uniquely increases the expression of anti-apoptotic gene programs and prevent inflammation-mediated apoptosis in hepatocytes.264 The expression of PGC-1α4 in vitro and in vivo induces skeletal muscle hypertrophy, while the loss of PGC-1α4 reverses this result. Importantly, transgenic expression of PGC-1α4 in muscle reduces the loss of muscle mass and strength and improves glucose homeostasis during cancer progression, thereby dramatically ameliorating cancer-induced cachexia.262 In addition, transgenic expression of PGC-1α4 in skeletal muscle induces VEGF in vivo, whereas the knockdown of PGC-1α4 abrogates the induction of angiogenesis in response to hypoxia.265 A recent investigation revealed that PGC-1α4 partially modulates the metabolic benefits of resistance exercise. Overexpressing PGC-1α4 enhances glucose uptake in mouse myotubes and promotes anaerobic glycolysis in a PPARβ- and AMPK-dependent manner.266 These studies have unveiled the important function of PGC-1α4 in regulating diverse cellular processes.
In response to resistance exercise, PGC-1α is reduced regardless of the training state.267 PGC-1α2 and PGC-1α3 show a similar induction pattern after acute resistance exercise, with the magnitude of the response exacerbated by training. PGC-1α4 is not responsive to acute resistance exercise, but is significantly induced in the trained state.267 Nevertheless, Ydfors et al. found that PGC-1α4 is upregulated by both endurance and resistance exercise in human skeletal muscle.268 Another study also indicated that acute resistance exercise, either performed alone or 6 h after aerobic exercise, upregulates PGC-1α4.269 These two observations suggested that PGC-1α splice variants does not appear to contribute to distinct adaptations to resistance or endurance exercise.268,269 Interestingly, in resistance-trained individuals, PGC-1α4 expression following a resistance exercise session has a triphasic pattern: it initially decreases below baseline levels at 45 minutes after exercise, then increases at 3 h post-exercise, and finally decreases below baseline levels again at 48 h post-exercise. Meanwhile, despite the changes in PGC-1α splice variant expression, total PGC-1α expression remains unchanged and then decreases following resistance exercise.270 More studies are needed to understand the effects of exercise on inducing different PGC-1α splice variants and the dynamic alteration of PGC-1α variants mRNA expression following exercise.
L-PGC-1α and B-PGC-1α
Apart from the alternative promoter located upstream to the original promoter, there is another promoter of PGC-1α gene (termed exon 1 L) in the human liver, which is located within intron 2, is described. The resulting protein, called L-PGC-1α, is identical to PGC-1α except for a deletion of 127 amino acids at the N terminus (encoded by exons 1, 2 and part of 3). The absence of N-terminal region prevents L-PGC-1α from recruiting SRC-1 and CREB-binding protein and interacting with GCN5. However, because of the reservation of C-terminal containing nuclear localization signal, L-PGC-1α is mainly located in the nucleus and coactivates PPARα, PPARγ, and HNF4α.271 Therefore, similar to PGC-1α, L-PGC-1α can enhance FAO and mediate hepatic gluconeogenesis by interacting with these coactivators, thus supporting hepatic ATP production in the fasting state.271 Besides, Yao et al. demonstrated that HCV infection upregulates both PGC-1α and L-PGC-1α, which further promotes HCV production. Specifically, HCV infection induces ER stress, which upregulates phosphorylated CREB and L-PGC-1α, finally in turn leading to the involvement in the RNA replication and assembly of HCV, eventually promoting HCV production.272
The transcription start site of brain-specific PGC-1α isoforms (B-PGC-1α) is located 587 kb upstream of exon 2.273 The full-length brain-specific transcripts contain the newly identified exons and reference gene exons 2–13 arranged in a regular order. Importantly, this novel promoter is active in neuronal cell lines, and haplotypes encompassing the novel promoter are more strongly associated with HD age of onset compared to previously described SNPs or haplotypes for the reference locus.273
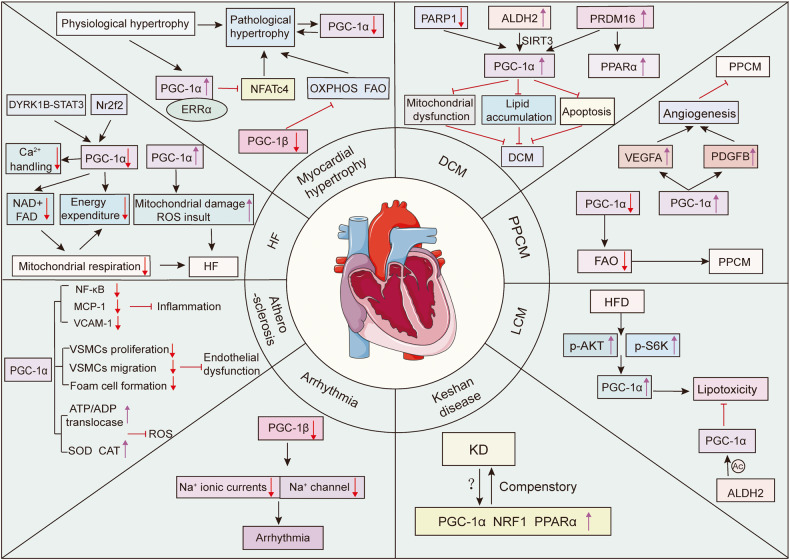
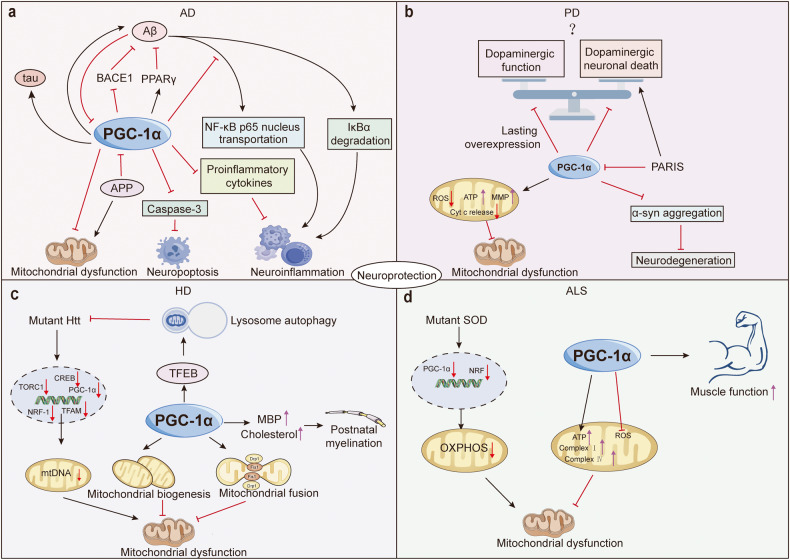
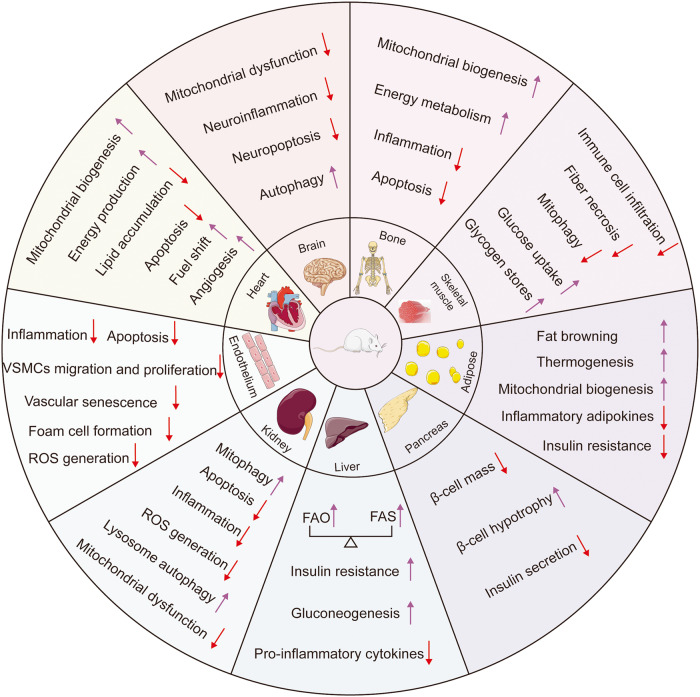
The role of Pgc-1s in pathophysiological processes and diseases
PGC-1s in cancers
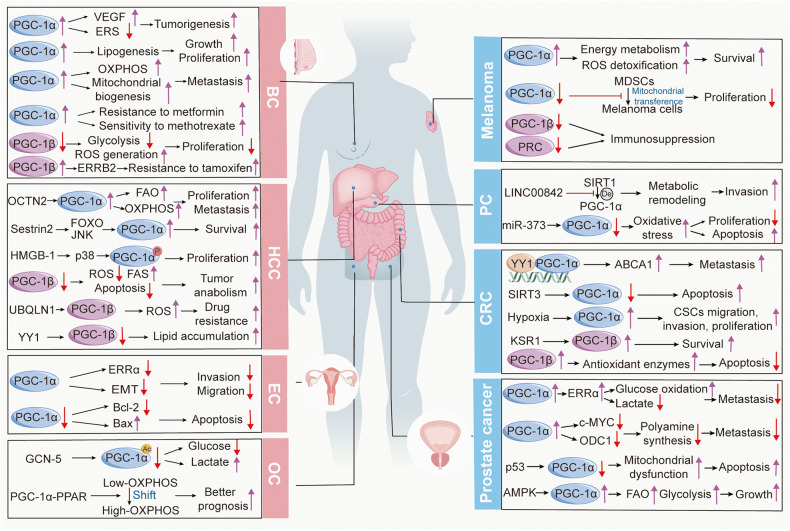
An array of studies suggests that PGC-1s are aberrantly expressed in a diverse range of cancer types and are implicated in tumor proliferation, migration, invasion, metastasis, drug sensibility and resistance, and adaptation to metabolic stress.274–277 These findings largely stem from that PGC-1s are irreplaceable central molecules in imperative cellular events involved in the development of cancer, including mitochondrial OXPHOS, nutrient anabolism and catabolism, autophagy, and apoptosis. Noticeably, PGC-1s exhibit different functions not only in distinct types of cancer but also in the same tumor, ranging from antitumor properties to advantageous for cancer cells. These observations imply that the roles of PGC-1s in cancer are both specific to the tissue or organ type and dependent on the particular physiological processes (Fig. 5). Therefore, conducting a systematic review to gather current opinions and future exploration to decipher more and deeper mechanisms are extremely significant for solving the therapeutic dilemma.
Fig. 5.
Mechanisms underlying the effects of PGC-1s in various cancers. PGC-1s are widely implicated in imperative cellular events involved in the development of cancers, including mitochondrial OXPHOS, nutrient anabolism and catabolism, autophagy, and apoptosis, and exhibit both detrimental and deleterious effects in cancers
Colorectal cancer
Although colorectal cancer (CRC) was infrequently diagnosed several decades ago, it has been the fourth most deadly cancer in the world, with almost 900, 000 deaths annually nowadays.278 Previous research primarily considered PGC-1α as a predictor of lymph node metastasis and poor prognosis in human CRC.279–281 Recently, accumulating compelling evidence has emphasized the sophisticated molecule network regarding the roles of PGC-1s in CRC.
In vitro and in vivo studies, PGC-1α knockdown restrains CRC cell proliferation, migration, invasion, and angiogenesis. Mechanistically, PGC-1α interacts with transcription factor YY1, further stimulating ATP-binding cassette transporter 1 (ABCA1) transcription and ABCA1-mediated cholesterol efflux, which aggravates epithelial-mesenchymal transition (EMT), ultimately facilitating CRC metastasis.282 Another downstream pathway of PGC-1α in CRC is AKT/GSK-3β/β-catenin.283,284 PGC-1α knockdown downregulates the expression of p-AKT, p-GSK-3β, β-catenin, N-cadherin and mitigates cell proliferation, migration, and invasion, while the opposite effects are observed in PGC-1α overexpressing cells.284 Moreover, PGC-1α can act as a downstream molecule of SIRT3 in CRC. Under oxidative stress, SIRT3 is recruited with PGC-1α, and suppressing SIRT3 decreases PGC-1α expression, leading to decreased mitochondrial activity and increased apoptosis in cells treated with anticancer drugs.285 Cancer stem cells (CSCs), a type of quiescent, pluripotent, self-renewing neoplastic cells, are recognized as tumor-initiating cells.286 The researchers discovered that PGC-1α is a master regulator of lactate oxidation and is elevated in normoxic CSCs. Further investigation revealed that PGC-1α mediates OXPHOS, thus promoting metastasis of normoxic colorectal CSCs.287 Hypoxia induces PGC-1α expression, which augments mitochondrial biogenesis, OXPHOS, antioxidant enzyme expression, migration, invasion, sphere formation, and proliferation and blocks apoptosis caused by the anti-cancer drug 5-fluorouracil in CRC cells, finally exacerbating tumorigenesis.288 Of note, when exposed to 5-fluorouracil, PGC-1α can also promote cancer cell survival via the modulation of mitochondrial function, ER stress, and the apoptotic signaling pathway.289
RAS mutations, including HRAS, NRAS, and KRAS, are among the most common oncogenes. The kinase suppressor of Ras 1 (KSR1) is necessary for Ras-induced tumorigenesis. Notably, PGC-1β, as a key downstream effector of KRAS and KSR1, is required for CRC survival both in vitro and in vivo.290 The same group further demonstrated that KSR1 protects erythropoietin-producing hepatocellular carcinoma receptor B4 (EPHB4) from lysosome-dependent degradation and increases Myc expression, which upregulates PGC-1β expression to expand the metabolic capacity of the cells and facilitate survival.291 Furthermore, overexpressing PGC-1β induces the expression of antioxidant enzymes and renders enterocytes less susceptible to ROS-driven macromolecule damage, thus leading to a delay in apoptosis and an increment in tumor susceptibility and growth rate when exposed to carcinogens.292
Collectively, PGC-1s, acting as gatekeepers of redox status and metabolic conditions, play promotive roles in CRC.
Hepatocellular carcinoma
Hepatocellular carcinoma (HCC), the fourth most common cause of cancer-related death worldwide, poses a significant global healthcare challenge.293 Yang et al. found that organic cation/carnitine transporter 2 (OCTN2) is significantly elevated in HCC and has a strong association with poor prognosis. Mechanistically, the upregulation of OCTN2 promotes the proliferation and migration of HCC cells in vitro and augments the growth and metastasis of HCC, as well as the cancer stem-like properties of HCC by increasing FAO and OXPHOS, which depends on PGC-1α signaling.294 When glucose deprivation occurs, sestrin2, a conserved antioxidant and metabolism regulator, stimulates a decrement in intracellular glutamine and PGC-1α levels, leading to a decline in cell survival. Further mechanistic experiments have revealed that sestrin2 forms a complex with c-Jun N-terminal kinase and FOXO1, thereby facilitating the nuclear translocation of FOXO1 and consequently promoting the transcription of PGC-1α.295 Additionally, in the diethylnitrosamine-induced HCC model, the genetic blocking of high mobility group box (HMGB)-1 slows tumor cell growth during hypoxia. The researchers further illuminated that HMGB1 translocates from the nucleus to the cytoplasm and binds to cytoplasmic Toll-like receptor, resulting in the activation of p38 and subsequent phosphorylation of PGC-1α, which upregulates mitochondrial biogenesis, finally promoting tumor survival and proliferation.296
Unlike PGC-1α, PGC-1β appears to be a double-edged sword in HCC. In one aspect, high level of PGC-1β boosts the expression of ROS scavenger and diminishes ROS accumulation and apoptosis. At the same time, it upregulates the expression of genes involved in FAS and triglyceride synthesis, thus supporting tumor anabolism.297 In another aspect, increased degradation of PGC-1β, triggered by UBQLN1, attenuates mitochondrial biogenesis and ROS production in sorafenib-resistant cells under sorafenib treatment, finally causing sorafenib resistance.298 Meanwhile, the inhibition of PGC-1β mediated by YY 1 attenuates both medium-chain and long-chain acyl-CoA dehydrogenase levels, leading to the suppression of FAO and exacerbating lipid accumulation, thereby driving HCC progression.299 These results reflected that PGC-1s, acting as the downstream targets of some molecules, exert both suppressive and promotive functions in HCC.
Breast cancer
Breast cancer (BC) is the most frequent invasive malignancy and the second leading cause of cancer-related deaths in females with an estimated 2.3 million new cases and >685,000 deaths.300 Remarkably, although mitochondrial respiration is the main biological function of PGC-1s, additional crucial roles of PGC-1s in glycolysis, glutaminolysis, angiogenesis, and detoxification contribute to its modulatory effects in BC.
Indeed, PGC-1α promotes the growth of ErbB2/Neu-induced mammary tumors by modulating nutrient availability. In vivo, PGC-1α positively regulates the angiogenic factor VEGF and glucose levels and reduces ER stress, thereby alleviating UPR and favoring tumorigenesis.301 In addition, glutamine has been reported to play a central role in lipid biosynthesis in cancer cells.302 The overexpression of PGC-1α and subsequent activation of ERRα modulates forward and reverses glutamine flux through the citric acid cycle, thereby boosting de novo lipogenesis reactions, particularly in hypoxic conditions, ultimately conferring growth and proliferation advantages to BC cells.303 These observations are also supported by the clinical data showing that PGC-1α expression is positively correlated with that of the glutamine pathway in ERBB2+ and high expression of this axis is associated with poor prognosis for BC patients.303 BC cells that preferentially metastasize to the lung or bone display relatively high expression of PGC-1α compared to those that metastasize to the liver. PGC-1α promotes BC cell migration and invasion in vitro and augments lung metastasis in vivo, which is linked to enhanced global bioenergetic capacity.304 As migratory/invasive cancer cells specifically prefer mitochondrial respiration and increased ATP production, it is not surprising that invasive cancer cells boost OXPHOS, mitochondrial biogenesis, and the oxygen consumption rate by enhancing PGC-1α to perform functional motility of cancer cells and metastasis.304–306 This is consistent with clinical analysis that a strong correlation between PGC-1α expression and the formation of distant metastases exists in invasive cancer cells.305 In terms of drug response, on the one hand, PGC-1α promotes resistance to metformin (a novel class of potential anti-cancer drugs referred to as energy disruptors) in BC metastasize to the lung cells.304 On the other hand, the PGC-1α/ERRα axis results in substantial perturbations in purine biosynthesis and the repression of one-carbon metabolism, which promotes the sensitivity of BC cells and tumors to the anti-folate drug methotrexate.307 Therefore, the true roles of PGC-1α in responding to drug therapy in BC remain elusive and require further investigation.
The evidence from the interaction between miRNA and PGC-1α also suggested that PGC-1α plays dual roles in BC. MiR-485-3p and miR-485-5p suppress BC cell metastasis by inhibiting PGC-1α expression. Specifically, overexpression of miR-485-3p and miR-485-5p suppresses mitochondrial respiration and potential for cell migration and invasion in vitro and also abrogates spontaneous metastasis of BC cells in vivo, which are partially relieved by restoration of PGC-1α expression.308 In addition, miR‑382 overexpression inhibits tumor‑associated macrophage polarization toward the M2 phenotype and M2‑type cytokine release that promotes EMT and the distant metastasis of BC cells, as well as the ability of tumor‑associated macrophages to promote the malignant behaviors of BC cells, while PGC‑1α expression weakens above changes.309 In contrast, miR-217-downregulation increases PGC-1α at both mRNA and protein levels and inhibits BC proliferation and cell-cycle progression, whereas siRNA-mediated PGC-1α downregulation reverses this phenomenon.79 Collectively, these observations reflect that PGC-1α plays both deleterious and beneficial roles in BC cell growth, proliferation, migration, and invasion.
Like PGC-1α, the functions of PGC-1β in BC appear to be paradoxical. It has been reported that the inhibition of PGC-1β decreases the glycolytic pathway, increases ROS generation, and impairs cell proliferation.310 Similarly, the suppression of PGC‑1β inhibits BC cell growth, proliferation, and migration, and promotes apoptosis by cooperating with the transcription factor FOXA2 or hexokinase domain component 1.311,312 Deblois et al. found that ERRα can be recruited to specific sites at chr.17q12 to regulate the expression of ERBB2 in human BC cells and PGC-1β is recruited to ERRα-bound segments in the chr.17q12 amplicon. The ERRα/PGC-1β complex then enhances the development of the ERBB2-positive tumor subtype and tamoxifen resistance in BC through transcriptional control of the ERRB2 amplicon.313 Moreover, the overexpression of miR-22-3p restrains the proliferation and migration of BC cells by directly targeting PGC-1β, ultimately regulating the PPARγ pathway in BC.314 However, miR-378 fulfils the metabolic shift that TCA cycle activity is reduced and the cells are less dependent on OXPHOS to fulfill their energy demands, which is achieved by suppressing the PGC-1β/ERRγ transcriptional pathway.315
Briefly, PGC-1s are of vital importance for BC progression by regulating multiple cellular and physiological processes. However, given the significant impact of BC to worldwide morbidity and mortality and conflictive results, further research is needed to fully comprehend the precise mechanisms underlying the involvement of PGC-1s in BC.
Ovarian cancer
OC is the most lethal gynecologic malignancy globally, characterized by poor prognosis and aggressive tumor growth.316 The specific molecular for early detection, disease risk stratification, and directing targeted therapies are significant. Research has discovered that PGC-1α/β expressions allow for patient stratification due to their association with the OXPHOS gene program and therefore may be potentially reliable biomarkers predictive of responsiveness to OXPHOS inhibitors in OC.317
As previously introduced, GCN5 is responsible for the acetylation of PGC-1α. In cyclin E1-driven OC, GCN-5/PGC-1α signaling is activated and associated with nutrient metabolism. Silencing of GCN5 genetically or pharmaceutically represses the acetylation of PGC-1α, decreases glucose uptake, and increases lactate production.318 Interestingly, the metabolomic analyses of frozen high-grade serous OC (HGSOC) samples from the Curie cohort revealed the existence of at least two subgroups with distinct metabolic profiles. High-OXPHOS HGSOC exhibits increased levels of cofactors involved in oxidation-reduction reactions, while low-OXPHOS HGSOC is featured by the accumulation of glutathione metabolism intermediates and choline intermediates. Importantly, PGC-1α-PPAR-mediated mitochondrial biogenesis is sufficient to promote the transition from low-OXPHOS to high-OXPHOS characteristics, which is associated with better prognosis in HGSOC patients. Mechanistically, PGC-1α localizes to subnuclear structures, facilitating its interaction with transcriptional cofactors and coregulators, in which the promyelocytic leukemia (PML) nuclear body constitutes an interface whereby PGC-1α interacts with transcriptional components. All these suggested that the PML protein-PGC-1α axis acts as one of the switches between high- and low-OXPHOS states by modulating the transcription of mitochondrial genes.319 In addition, silencing PGC-1α dramatically hinders invasion and migration in cyclin E1-driven OC cell lines.320
Endometrial cancer
Endometrial cancer (EC) accounts for approximately 76,000 deaths annually among women worldwide, with substantially increased incidence and mortality.321 In EC, PGC-1α performs a signaling orchestra with its coactivators, peculiarly ERRs, rather than functioning alone a single player itself. ERRα/PGC-1α overexpression increases the expression of EMT-associated factors including vimentin, Snail, and ZEB1 after exposure to TGF-β and reduces the expression of E-cadherin. However, ERRα knockdown suppresses TGF-β-induced migration and invasion in EC cells.322 The mRNA levels of PGC-1α and ERRγ are also positively connected with clinical staging, depth of myometrial invasion, and the number of metastatic lymph nodes in the endometrial adenocarcinomas.323 Additionally, the survival of EC cells is dependent on the synergism between PGC-1α and estrogen, which is achieved by the mitochondrial apoptotic pathway.324 Specific downregulation of PGC-1α expression promotes apoptosis in HEC-1A cell through the mitochondrial apoptotic pathway by downregulating the expression of Bcl-2 and upregulating the expression of Bax.325
Melanoma
Melanoma is one of the most common and aggressive skin cancers and continues to be a great contributor to cutaneous cancer-related mortality.326 It has been observed that two subpopulations of cells, one expressing high levels of PGC-1α and a second subpopulation with very low PGC-1α expression, exist in melanoma.327 Tumors expressing high levels of PGC-1α are associated with lower survival compared to tumors with low PGC-1α expression. Further mechanism research illuminated that mitochondrial energy metabolism and ROS detoxification capacities upregulate in PGC-1α high-expression melanoma cells, which enables melanoma cells to survive under oxidative stress conditions. Conversely, the melanoma cells expressing low PGC-1α levels are more glycolytic and vulnerable to ROS-inducing drugs.327 Intriguingly, the heterogeneous expression of PGC-1α within tumors leads to differences in their ability to proliferate or invade. Specifically, the population with low mitochondrial/PGC-1α activity tends to display a pro-metastatic gene program, while the population with high mitochondrial/PGC-1α activity drives a proliferation phenotype. This heterogeneity is critical for melanoma progression through changes in PGC-1α to respond to different signals, including nutrients, and switching between survival-proliferation and invasion-metastasis.328 Likewise, Gelato et al. supported the idea that melanoma models with elevated PGC-1α levels are characteristic by a proliferative phenotype.329
Amusingly, bone marrow-derived stromal cells (MDSCs) have the capacity to migrate to melanoma tumors. Melanoma proliferation is enhanced by acquiring mitochondria from tumor-supporting MDSCs, while the suppression of PGC-1α reduces mitochondrial transfer from MDSCs to melanoma.330 Besides, approximately 30.4-66.0% of cutaneous melanomas are attributed to BRAF mutation.331 The researchers illustrated that BRAF activation is associated with decreased oxidative enzymes, diminished mitochondrial quantity and function, and increased production of lactate and BRAF triggers this metabolic reprogramming via the suppression of PGC-1α and MITF, a melanocyte lineage factor.332
Noticeably, polymorphism studies revealed that PGC-1β rs32579 polymorphism is linked to tanning ability and provides protection from melanoma.333 Another exploration unveils the largely overlooked roles of PGC-1β and PRC in controlling inflammation and immunosuppression in melanoma. The global low expression of PGC-1s increases the expression of immunosuppressive cell surface proteins and cytokines, including galectin-9, PD-L1, PD-L, CD73, and IL-8.334 Simultaneously, the expression of PGC-1β and PRC transcripts decreases in tumors that do not respond to anti-PD-L1 therapy and the negative correlation between PGC-1β and PRC with immune genes is strong in the non-responder group. These analyses suggest that reduced expression of PGC-1s in melanoma impairs the response to immunotherapy, possibly through inducing a multigenic immunosuppressive transcription program.334
Collectively, these findings indicated that PGC-1s play indispensable roles in melanoma by influencing tumor phenotype, metabolic reprogramming, and immunosuppression.
Pancreatic cancer
Pancreatic cancer (PC) is currently one of the most lethal malignancies, with a five-year survival rate as low as 3%.335 The function of PGC-1s in PC has drawn extensive attention, mainly focusing on the interaction between PGC-1s and non-coding RNA. LINC00842 (a long intergenic noncoding RNA) has been shown to promote the progression and invasiveness of pancreatic ductal adenocarcinoma (PDAC) by targeting PGC-1α. Specifically, LINC00842 curbs acetylated PGC-1α from deacetylation by SIRT1, resulting in metabolic remodeling of PDAC cells, as exhibited by the transition from cellular mitochondrial oxidative catabolic processes to FAS.336 Moreover, miR-373 negatively regulates the expression of SIRT1 by directly binding to its 3’-UTR. Importantly, miR-373 restrains PC cell proliferation but exaggerates apoptosis through modulating oxidative stress response via SIRT1/PGC-1α/NRF2 axis.337
Besides, PC stem cells exhibit a distinct metabolic phenotype, which strongly depends on the mitochondrial OXPHOS, whereas non-CSCs mostly require glycolysis. The metabolic phenotype of CSCs is mainly determined by the Myc/PGC-1α ratio.338 Considering our current limited knowledge regarding the PGC-1s family in PC, more attention should be paid to elucidating the underlying modulatory mechanisms.
Prostate cancer
Prostate cancer remains the most frequently diagnosed non-skin malignancy that affects men’s health and 1 in 25 men globally is diagnosed with this malignant condition during their lifetime.339 According to data from the TCGA cohort, several well-established factors, that are associated with prostate cancer progression risks, have been identified, notably PPARGC1A.340 It is worth noting that PGC-1s also act as a double-edged sword in prostate cancer.
Some research provides new ideas and evidence supporting the therapeutic targeting of the PGC-1s-ERRs axis in prostate cancer.341 PGC-1α expression elicits an obvious decrement in the migratory capacity of PC3 and DU145 cells and a robust anti-invasive phenotype, but ERRα deletion abolishes the induction of target genes of the transcription factor upon induction of PGC-1α.341 Equally, PGC-1α activates an ERRα-dependent transcriptional program to control the balance between catabolic and anabolic processes, as shown by the increased glucose oxidation and reduced extracellular lactate levels in PGC-1α expressing cells, thereby exerting a potent anti-metastatic property.342 Furthermore, PGC‑1α restrains the metastatic properties of prostate cancer cells by regulating the polyamine biosynthesis pathway. Mechanistically, PGC‑1α inhibits the expression of c-Myc through an ERRα-dependent manner and ornithine decarboxylase 1 (ODC1), the rate-limiting enzyme for polyamine synthesis, further regulating polyamine biosynthesis and prostate cancer aggressiveness.343 These results support that PGC-1α-ERRα functions as a tumor-repressive transcriptional complex through modulating metabolic events. p53 is a tumor suppressor gene with extensive and powerful functions, known as the “guardian of the genome”.344 Li et al. found that p53 downregulates the expression and nuclear localization of the PGC‑1α protein and stimulates mitochondrial dysfunction, which promotes apoptosis, highlighting PGC‑1α as an essential target of p53-induced apoptosis in prostate cancer cells.345
Nevertheless, in contrast to these, the PGC-1s pathway has been demonstrated to promote prostate cancer cell growth.346,347 On the one hand, PGC-1α interacts with the N-terminal domain of androgen receptor (AR), participates in the N- and C-terminal interaction of AR, and upregulates the DNA-binding ability of AR to androgen-responsive elements in the prostate-specific antigen enhancer and promoter regions to increase the transcription of AR target genes, finally facilitating prostate cancer cell growth.346 On the other hand, prostate cancer cells respond to androgen treatment by increasing glycolysis rates, glucose, and FAO, which is dependent on androgen-mediated AMPK activity and subsequent PGC-1α activation. In other words, androgens regulate prostate cancer cell growth via an AMPK-PGC-1α-mediated metabolic switch.347
PGC-1s in noncancer diseases
PGC-1s in cardiac diseases and cardiovascular diseases
The connection between the PGC-1s pathway and the cardiovascular system has been investigated since it was discovered. As early as 2000, Lehman et al. identified PGC-1 as an essential regulatory molecule in the control of cardiac mitochondrial number and function in response to energy demands.3 Subsequently, a series of studies revealed that PGC-1s play indispensable roles in mediating cardiac fuel transport and consumption, energy state, and the development and function of the heart.348–352 For example, PGC-1α expression in the heart significantly increases at birth, which is required for a high-level expression of nuclear and mitochondrial-encoded genes involved in mitochondrial energy transduction and OXPHOS, and for full respiratory capacity.160 Therefore, dysregulation of the PGC-1s pathway substantially disrupts cardiac metabolism homeostasis and results in different types of cardiac diseases and cardiovascular diseases (Fig. 6).
Fig. 6.
The roles of PGC-1s in cardiac diseases and cardiovascular diseases. (1) In HF, PGC-1α deficiency causes major alterations in mitochondrial respiration and growth, ultimately giving rise to cardiac dysfunction. (2) The expression and activity of PGC-1α initially increase to meet the energy requirements during physiological hypertrophy, but consistently elevated levels of PGC-1α further leads to pathological hypertrophy. Meanwhile, PGC-1β deficiency aggravates the transition from hypertrophy to HF. (3) The upregulation of PGC-1α induced by several upstream molecules restrains DCM development by mediating lipid metabolism, mitochondrial function, and apoptosis. (4) PGC-1α can affect PPCM in three ways: 1) triggering the pro-vascular VEGF-mediated angiogenic signaling; 2) meeting the need for a fuel shift towards FAO;and 3) regulating energy metabolism. (5) HFD-induced reduction in PGC-1 provokes cardiac lipotoxicity. In contrast, PGC-1 overexpression counteracts the fat accumulation and heart defects induced by HFD. (6) The mRNA levels of PGC-1α, NRF1, and PPARα shows compensatory increase in KD, but precise regulatory molecular mechanisms of PGC-1s in KD is unkown. (7) PGC-1β deficiency leads to aberrant Na+ ionic currents and Na+ channel, then enhancing arrhythmic ventricular phenotype. (8) The mechanisms that PGC-1α represses atherosclerotic disease progression involves in inhibiting ROS, endothelial dysfunction, and inflammation
PGC-1s in heart failure
Heart failure (HF), the most devastating consequence of cardiovascular disease, is characterized by variable durations of symptomatic stability even worsening symptoms despite continued therapy.353 A variety of research indicated that the mRNA and protein levels of PGC-1s and its coactivator as well as the target genes are downregulated in HF patients.354–356 Notably, serum PGC-1α is inversely correlated with energy expenditure and PGC-1α level reflects the degree of myocardial energy expenditure and the systolic function of the left ventricle in patients with chronic HF.357 In a cohort of 35 consecutive stable HF patients with severe aortic stenosis who underwent an elective aortic valve replacement surgery, a higher systemic PGC-1α expression is associated with higher SIRT1 levels and Trolox concentration, suggesting a better antioxidant status in these patients. Therefore, PGC-1α can be used as prognostic indicator in cardiovascular diseases.358 However, different study groups detected unchanged protein levels of PGC-1α in HF.359,360 These contradictory results might be explained by differences in the time point tested and sample diversity. Defining the complete mapping of expression changes of PGC-1α during the whole progression of HF will contribute to more precise therapy.
Some convincing evidence from genetic deletion animal models further supports the critical roles of PGC-1α in HF. For instance, PGC-1α-/- mice display profound cardiac dysfunction in response to cardiac duress, as initiated by constriction of the transverse aorta.361 The metabolome analysis revealed that heart-specific knockout of PGC-1α leads to major alterations in the metabolic processes associated with mitochondrial respiration and growth, as demonstrated by the reduced levels of acetyl-CoA, NAD + , FAD, acylcarnitine, and succinic acid, eventually causing HF.362 In addition, PGC-1α dysregulation abrogates the recruitment of RNA Polymerase II to metabolic gene promoters, thus inducing HF phenotypes.359 Likewise, Naumenko et al. observed that PGC-1α deficient mice develop dilated HF associated with suppression of energy metabolism, compromised calcium handling of cardiomyocytes, and remodeling of electrophysiological properties of cardiomyocytes. Interestingly, they further found more rapid and drastic contractile dysfunction and earlier death in female mice compared with male, suggesting that maintenance of normal phenotype and function are more reliant on intact energy metabolism in female than male hearts.363 In addition, PGC-1α also mediates the protective role of nuclear receptor subfamily 2-group F-member 2(Nr2f2) and DYRK1B deletion, validating the potential possibility of targeting PGC-1α for HF therapy.364,365
Nevertheless, several other studies manifest that the excessive expression of PGC-1α does not exert a beneficial role and even facilitates the development of HF. Karamanlidis et al. used a transgenic mouse model of moderate overexpression of PGC-1α ( ~ 3-fold) in the heart and found that PGC-1α upregulation does not improve cardiac energetics and function. Long-term overexpression of PGC-1α renders mice more vulnerable to acute cardiac stress and mice fails to protect against cardiac dysfunction caused by chronic pressure overload.366 In addition, cardiac-specific overexpression of PGC-1α ameliorates mitochondrial and cardiac function in 3-month-old WT mice but facilitates cardiac aging and markedly shortens lifespan in 12-month-old WT mice due to increased mitochondrial damage and ROS insult.367 In summary, owing to the complexity of the signaling pathway and the importance of maintaining cardiac homeostasis, it is necessary to carefully consider and explore the range and period of regulating PGC-1α levels.
PGC-1s in myocardial hypertrophy
Myocardial hypertrophy is an adaptive response to physiological and pathological overload. When exposed to overload, activated intracellular hypertrophic signaling pathways facilitate myocardial angiogenesis to dissolve the hypoxic situation and to maintain cardiac contractile function, but sustained overload induces pathological hypertrophy, generally progressing to HF.368,369 Growing compelling evidence suggested that PGC-1α is a multifaceted regulator in both physiological and pathological forms of myocardial hypertrophy. Under physiological conditions of increased energy demand, including exercise and fetal heart development, the elevated level of PGC-1α promotes mitochondrial biogenesis and ameliorates energy metabolism.370,371 In contrast to this, during pathological myocardial hypertrophy, the expression of PGC-1α is downregulated, which is also associated with a net loss of mitochondrial protein and oxidative capacity.372,373
In triiodothyronine (T3) induced cardiac hypertrophy, the mRNA level of PGC-1α decreases first and subsequently increases, but the overexpression of PGC-1α improves cardiac function through increasing energy production and mitochondrial biogenesis. Thus, it is possible that PGC-1α increases via an indirect or compensated mechanism.374 Liu et al. revealed the protective mechanisms of PGC-1α on myocardial hypertrophy. PGC-1α represses the expression of calcineurin-nuclear factor of activated T cells c4 (NFATc4) that participates in the regulation of heart development and bioenergetics, prevents its dephosphorylation and nuclear translocation, and further abrogates its binding activity to brain natriuretic peptide promoter, ultimately protecting cardiomyocytes from hypertrophy.375 In addition, the injection of AAV9-anti-miR-199a tough decoys virus alleviates cardiac hypertrophy and restores cardiac function, which depends on the PGC-1α/ERRα axis.77 Noticeably, a recent investigation demonstrated that PGC-1α expression in the physiological range in pressure overload hypertrophy (POH) preferentially preserves angiogenesis but is not sufficient to prevent POH-induced mitochondrial or contractile dysfunction.376 Collectively, facilitating PGC-1α signaling plays a cardioprotective role against pathological myocardial hypertrophy.
As for another member, PGC-1β expression is also diminished in POH. In the transverse aortic constriction model, PGC-1β deficiency aggravates oxidative stress, decreases cardiac efficiency, glucose metabolism, and hexokinase II protein, further accelerating the transition to HF, while PGC-1β activation mediates the protective roles of melatonin and attenuates cardiac contractile function.377,378 Considering that there are few studies on PGC-1β or PRC in cardiac hypertrophy and fibrosis, further research is needed in the future.
PGC-1s in cardiomyopathy
Cardiomyopathy refers to cardiac dysfunction caused by various factors, such as diabetes, pregnancy, and obesity.379–381 This section discusses how PGC-1α plays a vital role in these different types of cardiomyopathy.
Diabetic cardiomyopathy (DCM), resulting from insulin resistance, T2DM, and associated hyperinsulinemia independent of hypertension and coronary heart disease, is a major cause of morbidity and mortality in developed nations.382,383 Recent studies have suggested that PGC-1α and its coactivators play regulatory roles in DCM development by mediating lipid metabolism, mitochondrial function, antioxidant defense, and insulin resistance.384–386 Mitochondrial aldehyde dehydrogenase (ALDH) 2 serves as an imperative cardioprotective molecule against insulin resistance-induced cardiomyopathy, which is closely linked to the promotion of the SIRT3-dependent PGC-1α deacetylation.387 The transcription factor PR-domain containing 16 (PRDM16) is another protective factor in DCM. PRDM16 cardiac-specific deficiency mice manifest worsened cardiac dysfunction, aggravated mitochondrial dysfunction, cardiac lipid accumulation, and apoptosis. Co-IP and luciferase assays confirmed that PRDM16 regulates the transcriptional activity, expression, and interaction of PPARα and PGC-1α, while the overexpression of PPARα and PGC-1α reverses PRDM16 deficiency-induced cellular dysfunction in T2DM model. All these suggested the critical effects of PPARα and PGC-1α in PRDM16-mediated cardioprotective action.386 Besides, in the development of DCM, PGC-1α activation is responsible for reversing the Warburg effect to aerobic respiration when exercising, thus enhancing mitochondrial metabolism and energy homeostasis.388
Peripartum cardiomyopathy (PPCM) occurs globally and is accompanied by systolic dysfunction that presents in late pregnancy or, more commonly, the early postpartum period.389 Mice lacking cardiac PGC-1α develop profound PPCM, as shown by enlarged left ventricular end-diastolic dimensions and left ventricular end-systolic dimensions, and depressed cardiac contractile function.390 However, overexpression of PGC-1α in neonatal rat ventricular myocytes (NRVMs) strongly increases angiogenic genes involved in the activation and recruitment of endothelial cells (including VEGFA) and mural cells (including PDGFB), as well as genes that take part in the mitochondrial respiratory chain (including Cycs and Cox5b), suggesting that PGC-1α controls an angiogenic program, which entirely rescues PPCM.390 Conversely, β1-Adrenoceptor antibodies-treated postpartum rats manifest PPCM, which is associated with the repression of PGC-1α in parallel with the decline of its downstream transcript VEGF.391 Garcia and colleagues have found that methyl donor deficiency aggravates the metabolic condition of PPCM. Specifically, the methyl donor deficiency leads to imbalanced methylation/acetylation of PGC-1α and decreased expression of PPARα and ERRα, further causing detrimental effects on FAO and energy metabolism.392 In addition, PGC-1α and its coactivated partners PPARs play principal roles in the regulation of FAO as discussed above.28,393 Because of an increasing fuel shift towards high reliance on FAO in the gestational heart,394 aberrant FAO can contribute to PPCM. Generally, PGC-1α can affect PPCM in three ways: 1) triggering the pro-vascular VEGF-mediated angiogenic signaling; 2) meeting the need for a fuel shift towards FAO; and 3) regulating energy metabolism.
As previously introduced, PGC-1α plays an important role in regulating lipid metabolism. Therefore, it has a close connection with obesity cardiomyopathy and lipotoxic cardiomyopathy (LCM). HFD intake induces weight gain, hypertrophy and interstitial fibrosis, contractile dysfunction, mitochondrial injury, and apoptosis, whereas ALDH2 offers protection against HFD-induced cardiomyopathy through reversing the changes in CaMKII, SIRT1, and PGC-1α acetylation.395 In line with this, HFD-induced reduction in PGC-1/spargel (srl) expression provokes cardiac lipotoxicity. HFD feeding activates TOR signaling (increased p-AKT and p-S6K), which in turn gives rise to the downregulation of PGC-1/srl expression. In contrast, PGC-1/srl overexpression counteracts both the fat accumulation and heart defects induced by HFD. These findings identified an integrated genetic network for counteracting obesity and associated cardiac lipotoxicity, in which PGC-1 is both necessary and sufficient.396
In addition, mitochondrial-related gene expression profiles reflect important roles of PGC-1α in the compensatory mechanism of Keshan disease (KD), an endemic dilated cardiomyopathy with unclear etiology. The researchers found that six nuclear receptor-related pathways and eight genes, as well as four energy production-related pathways and five genes are upregulated in KD and PGC-1α-induced energy production plays an important role in the compensatory mechanism of KD.397 Recently, Jiang et al. discovered that the mRNA levels of PGC-1α, NRF1, and PPARα are higher in patients with KD. Notably, the area under the curve for the “lactate dehydrogenase (LDH) + PPARα” combination was 0.984, with 96.7% sensitivity and 93.0% specificity, indicating that the combined detection of LDH and PPARα can be performed to diagnose chronic KD.398 Nevertheless, our current knowledge of PGC-1s in KD and precise molecular mechanisms is incomplete, and additional work is needed in the future.
PGC-1s in arrhythmia
Different from other diseases, PGC-1β rather than PGC-1α has drawn widespread attention in arrhythmia. PGC-1β deficient mice show pro-arrhythmic ventricular phenotype secondary to mitochondrial dysfunction.399–401 In addition, cardiomyocyte Na+ ionic currents in the age-dependent murine PGC-1β model of ventricular arrhythmia are reduced.402 Compared to WT, the protein expressions of the Na+ channel in murine PGC-1-/- atria are also reduced.403 These changes suggest potential roles of PGC-1β in cardiac electrophysiology and ion channel changes. However, compared to research on PGC-1s in other cardiac diseases, the current understanding of PGC-1s in arrhythmia is only at the tip of the iceberg and is far from adequately sufficient to describe the specific role of PGC-1s in arrhythmia. Thus, further efforts are warranted to fully elucidate PGC-1s involved in the pathologic mechanisms of arrhythmia.
PGC-1s in atherosclerosis
Atherosclerosis is a chronic inflammatory and lipid-depository disease of the arterial wall and is a leading cause of acute cardiovascular events and death worldwide.404 One case-control survey reported that Gly482Ser polymorphism in the gene encoding PGC-1α contributes to the risk of coronary artery disease.405 Meanwhile, the PGC-1α protein is markedly downregulated in human atherosclerotic vessel samples.80 These remind the potential effects of PGC-1α in atherosclerosis. Next, we further describe the crucial roles of PGC-1s in inflammation, oxidative stress, endothelial cell dysfunction, and vascular smooth muscle cells (VSMCs) activities during atherosclerosis.
ROS production is the main cause of endothelial cell injury as ROS increase endothelial permeability, promote leukocyte adhesion, and change endothelial gene expression.406 Indeed, the powerful induction of PGC-1α in antioxidant proteins greatly contributes to its action in atherosclerosis. TNF-α, a major proinflammatory factor in vascular inflammation, increases intracellular ROS production. Overexpression of PGC-1α in human aortic smooth (HASMCs) and endothelial cells (HAECs) reverses the above phenomenon and suppresses NF-κB activity, and monocyte chemoattractant protein-1 (MCP-1) and vascular cellular adhesion molecule-1 (VCAM-1) expression induced by TNF-α, thus preventing the development of atherosclerosis.407 In addition, PGC-1α can enhance ATP/ADP translocase activity and nicotinamide adenine dinucleotide phosphate (NADPH) oxidase degradation through proteasome degradation pathway, further restraining ROS generation and apoptosis in endothelial cells.408,409 C1q/TNF-related protein-9 ameliorates oxidized low-density lipoprotein (ox-LDL)-induced endothelial dysfunction, which is mediated by PGC-1α/AMPK-induced antioxidant enzyme.410
It is known that VSMCs proliferation is detrimental throughout atherosclerosis.411 Accumulating evidence highlighted that PGC-1α protects VSMCs from proliferation, migration, and inflammation.412–414 For example, free fatty acids, including oleic acid and palmitic acid, stimulate VSMCs proliferation and migration and result in the formation of organized atherosclerotic plaque. PGC-1α overexpression blocks VSMCs proliferation and migration due to its capacity to prevent ERK phosphorylation, while the suppression of PGC-1α by siRNA enhances the effects of oleic acid and palmitic acid.415,416 The regulator of lipid metabolism perilipin 5 (Plin5) knockdown leads to accelerated neointima hyperplasia, excessive proliferation, and migration of VSMCs and inhibits the interaction between plin5 and PGC-1α. Importantly, researchers further illustrated that overexpression of PGC-1α suppresses ROS generation, proliferation, and migration in VSMCs.417 The process by which monocytes differentiate into macrophages and macrophages recognize and take up highly ox-LDL particles, which can lead to foam cell formation, is considered one of the vicious points, finally causing atherosclerotic plaque.418 Notably, PGC-1α is localized to macrophage/foam cells in the murine aorta where its expression is increased when conjugated linoleic acid attenuates murine atherosclerosis. Overexpression of PGC-1α in bone marrow-derived macrophages diminishes foam cell formation, whereas macrophage-specific deletion of PGC-1α accelerates atherosclerosis in the LDLR-/- mouse.419 Methyl-transferase-like 3 (METTL3) acts during ox-LDL-induced monocyte inflammation. Mechanistically, METTL3 and YTH N6-methyladenosine RNA binding protein 2 cooperatively modify PGC-1α mRNA, regulate PGC-1α degradation, and downregulate PGC-1α protein levels, thereby enhancing the inflammatory response.420
Aging is considered an independent risk factor for human atherosclerosis and vascular senescence facilitates plaque vulnerability, which greatly increases the possibility of cardiovascular events.421,422 Xiong et al. identified PGC-1α as a negative regulator of vascular senescence in vivo and in vitro. Angiotensin II leads to SIRT1 and CAT downregulation and vascular senescence, which is achieved by inducing prolonged lysine acetylation of PGC-1α and interrupting the PGC-1α-FOXO1-SIRT1 feed-forward.419 PGC-1α deficiency can also mediate impaired autophagy caused by the downregulation of SQSTM1 (autophagy receptor), thus accelerating vascular aging and atherosclerosis.423
In conclusion, the roles of PGC-1α in atherosclerosis have gained extensive attention. PGC-1α deficiency in endothelial cells, VSMCs, and monocytes/macrophages promotes atherosclerosis. Hence, PGC-1α might be a potential therapeutic target for the treatment of atherosclerosis.
PGC-1s in neurological disorders
Neurological disorders, especially neurodegenerative diseases (NDs), are characterized by progressively structural and functional loss of neurons in discrete areas of the central nervous system (CNS), accompanied by memory difficulty, uncontrolled motor activities, and impairment in expressive speech, visuospatial processing, and executive functions, posing looming dire economic and societal impacts.424 The more common NDs in the elderly population are Alzheimer’s disease (AD), Parkinson’s disease (PD), Huntington’s disease (HD), and amyotrophic lateral sclerosis (ALS). Given the high metabolic demand of the brain, and the importance of ATP synthesis and the maintenance of mitochondrial function for neuronal activity, PGC-1s have been extensively studied as a center in the network of energy metabolism. Analysis of PGC-1α expression patterns showed that PGC-1α is abundantly expressed in the brain areas, such as the cerebral cortex, hippocampus, striatum, thalamic nucleus, and substantia nigra.425 PGC-1α is also implicated in maintaining cholinergic,426 glutamatergic,427 dopaminergic,428–430 and GABAergic synapses.431,432 PGC-1α deficiency in specific brain areas, including GABAergic neurons causes short-term habituation, hyperactivity, and exaggerated startle reactivity.433 On the other hand, activation or overexpression of PGC-1α can counteract neurological disorders by improving mitochondrial function, neuronal maintenance, neuroinflammation, and protein clearance.434–436 Herein, we will provide a complete picture of the role of PGC-1s in different models covering AD, HD, PD, and ALS (Fig. 7), aiding in the design of future studies and advancing investigations of PGC-1α as a therapeutic target in the nervous system diseases treatments.
Fig. 7.
Mechanisms of neuroprotection mediated by the PGC-1α signaling network. a PGC-1α inhibits Aβ deposition, neuroinflammation, neuropoptosis, and mitochondrial dysfunction, but it also exacerbates Aβ and tau accumulation in AD. b PGC-1α overexpression represses dopaminergic neuronal loss, behavioral deficits, mitochondrial dysfunction, and neurodegeneration, while its lasting overexpression suppresses dopaminergic function in PD. c PGC-1α upregulation promotes HTT protein elimination and postnatal myelination, and inhibits mitochondrial dysfunction. d PGC-1α upregulation increases ATP production and enhances muscle function in ALS
PGC-1s in AD
AD, featured by progressive impairment in cognition, emotion, language, and memory in older population, is an irreversible, multifactorial, and age-related neurodegenerative disease.437 A putatively fatal etiological hypothesis is the accumulation of Aβ.438 Importantly, there are complicated and direct links between PGC-1α and Aß. Amyloid precursor protein (APP)/PS-1 transgenic mice are popular animal models of AD. BACE1 is the main enzyme involved in Aβ generation. Four months after injection of PGC-1α in APP23 mice, improved spatial and recognition memory concomitant with a significant reduction in Aβ deposition and decreased expression in BACE1 are observed.439 The findings by other teams that PGC-1α activation or overexpression severely diminishes the protein expression of BACE1 and Aβ plaques also support the results.440–442 In addition, PGC-1α blocks Aβ generation through a PPARγ-dependent mechanism.110
Beyond Aβ deposition, emerging evidence strongly suggested that neuroinflammation and mitochondrial dysfunction are prerequisites for AD pathogenesis.443,444 Sheng and colleagues showed that expression levels of PGC-1α, NRF-1, and NRF-2 are significantly decreased in both AD hippocampal tissues and APPswe M17 cells. Overexpression of PGC-1α completely rescues, while knockdown of PGC-1α exacerbates impaired mitochondrial biogenesis and deficits in APP mutant M17 cells.445 Interestingly, the mRNA expression levels of CREB, PGC-1α, NRF-1, NRF-2, and TFAM are decreased as early as 1 month of age when there is no significant Aβ oligomer deposition in 3xTg-AD mouse (harboring PS1, APP, and tau human transgenes). At later ages, the protein expression of complex II, III, and IV and the activity of complex IV downregulate. These suggest that mitochondrial biogenesis is likely impaired in the ages preceding the development of AD pathology and is related to mitochondrial dysfunction at later ages.446 In addition, overexpression of PGC-1α remarkably reduces the level of pro-inflammatory cytokines and dampens the transportation of NF-κB p65 from cytoplasm to nucleus and IκBα degradation induced by Aβ1-42, implying that PGC-1α protects neuroblastoma cells against Aβ-induced neuronal death and neuroinflammation.447 From a therapeutic perspective, enhancing PGC-1α levels to boost mitochondrial biogenesis at early stages is a promising pharmacological approach for preventing the onset of AD. However, Dumont et al. illuminated that overexpressing PGC-1α in Tg19959 transgenic mouse exacerbates Aβ and tau accumulation, accompanied by an impairment of proteasome activity.448 These paradoxical conclusions reflect that maintaining the delicate balance between PGC-1α expression and its function plays crucial roles in the inhibition of AD and contributes to the design of treatments.
PGC-1s in PD
PD, a neurological disorder with evolving layers of complexity, features classical motor dysfunction associated with Lewy bodies (LBs) and dopaminergic neuron loss in the substantia nigra.449 Accumulating research illuminated that PGC-1α is involved in the regulation of these deadly physiological processes.
The cardinal motor symptoms of PD correlate with dopaminergic axonal neurodegeneration starting at the striatum, which is then followed by dopaminergic neuronal death in the substantia nigra pars compacta, resulting in dopamine deficiency.450,451 Previous studies have illustrated that knockdown of PARIS, a KRAB and zinc finger protein, leads to the mitochondrial respiratory decline and selective loss of dopamine neurons in the substantia nigra. This requires PARIS-induced downregulation of PGC-1α, owing to its ability to directly and endogenously occupy the cis-regulatory elements of PGC-1α.452,453 More recently, farnesol has been advocated as a PARIS repressor and it induces the farnesylation of PARIS, further eliminating its DNA binding affinity and preventing its suppression of PGC-1α, thereby antagonizing dopaminergic neuronal loss and behavioral deficits in PD.454 The researchers further demonstrated that increased PARIS ubiquitination and proteasomal degradation relieve its repressive effect of PGC-1α, thus alleviating mitochondrial biogenesis.455 These indicated that modulating the PARIS-PGC-1α pathway to promote mitochondrial biogenesis and inhibit the loss of dopamine neurons is beneficial in PD. Moreover, a series of studies also confirmed that 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) induces mitochondrial dysfunction and ROS production, as shown by decreased MMP and ATP levels, as well as increased H2O2 levels and release of cytochrome c, whereas PGC-1α overexpression partially reverses above phenomenon, thereby alleviating striatal loss of dopamine and progressive impairment of motor coordination. However, PGC-1α deficiency is opposite.428,430,456–462 However, there are discrepancies between different studies. Lasting overexpression of PGC-1α contributes to major alterations in the metabolic activity of neuronal cells, which dramatically impairs dopaminergic function, reduces striatal DA content, and enhances susceptibility to MPTP.429,463 Sometimes a compensatory loop exists between different molecules due to the artificial manipulation of the key components or regulation of the pathway, which may not precisely imply the real-world conditions. Therefore, while determining the role that PGC-1α plays in dopaminergic neuronal, researchers should also target its mechanism of action in order to lay the foundation for subsequent clinical translational studies.
Another histopathological hallmark of PD is the presence of fibrillar aggregates referred to as LBs containing α-synuclein (α-syn).464 PGC-1α null nigral neurons are more prone to degenerate following α-syn overexpression.465–467 In contrast, pharmacological activation or genetic overexpression of PGC-1α reduces α-syn oligomerization and α-syn-mediated toxicity.466 Additionally, in a zebrafish model of α-syn toxicity, overexpressing of PGC-1α in peripheral sensory neurons inhibits both cell death and axonopathy, thus protecting neurons from α-syn-induced toxicity.468 In conclusion, current studies have successfully highlighted the critical role of PGC-1α in the physiology of PD. However, at the molecular level, more exploration is required.
PGC-1s in HD
HD is the most frequent autosomal dominant neurodegenerative disorder resulting from an abnormally expanded CAG repeat expansion in the huntingtin (HTT) gene, which confers a predominant toxic gain of function in the mutant HTT protein.469 Remarkably, PGC-1α is downregulated in patients with HD and genetic repression of PGC-1α by mutant HTT increases striatal neurodegeneration and motor coordination in mice.470,471 Meanwhile, its upstream modulators, including TORCs and downstream transcription factors, such as NRF-1 and TFAM, are also downregulated.472,473
At the molecular level, PGC-1α stimulates TFEB, a master regulator of the autophagy-lysosome pathway, thereby promoting HTT protein turnover and elimination.474,475 At the organelle level, several publications advocated the role of PGC-1α in HD-related mitochondrial impairment and its potential as a therapeutic target to treat HD.470,476–478 PGC-1α upregulation increases mitochondrial mass and rebalances mitochondrial dynamics as well as promoting the mitochondrial fusion.477 In BAT from HD mice, a decrement in the numbers of functional mitochondria and ATP/ADP ratio are found. Combined with reduced expression of PGC-1α target genes involved in energy production in BAT, reduced PGC-1α activity possibly leads to a global defect in mitochondrial function in HD.470 At the tissue level, PGC-1α plays a role in postnatal myelination by regulating the expression of myelin basic protein (MBP) and cholesterol synthesis in HD. Decreased expression of MBP and deficient myelination are found postnatally in both adult HD models and PGC-1α knockout mice, whereas PGC-1α overexpression increases MBP promoter activity.479 These findings raise a possibility that upregulating PGC-1α activity may represent a novel strategy for early therapeutic interventions in HD.
PGC-1s in ALS
ALS is a fatal CNS neurodegenerative disease, characterized by the degeneration of both upper and lower motor neurons, which leads to muscle weakness and eventual paralysis.480 Notably, in both ALS animal models and ALS patients, the expression of PGC-1α and key mitochondrial genes (e.g. NRF1, NRF2, and TFAM) are downregulated.481–483 Liang and colleagues used PGC-1α transgenic mice to cross with SOD1 mutant G93A DL mice and revealed that PGC-1α/G93A DL mice exhibit markedly improved motor activity as compared with G93A DL mice, which is associated with a decreased loss of motor neurons and less degeneration of neuromuscular junctions.484 Elevated PGC-1α activity has been validated to sustain mitochondrial biogenesis and muscle function. PGC-1α expression increases mitochondrial energy-producing capacity, thereby making more ATP available for sustained muscle activity.485 PGC-1α overexpression dramatically improves motor function and survival, accompanied by reduced blood glucose level and by the protection of motor neuron loss, restoration of mitochondrial electron transport chain activities, and inhibition of stress signaling in the spinal cord.486 So far, there are relatively few explorations on the detailed underlying mechanisms regarding PGC-1α in ALS, substantially more studies should be initiated in the future.
PGC-1s in kidney diseases
The kidney requires abundant mitochondria to generate energy, thus achieving its inherent and specific tasks, from removing waste from the blood, and reabsorbing nutrients to maintaining fluid and electrolyte balance and regulating blood pressure.487 Increasing evidence suggests that dysfunctional renal mitochondria are pathological mediators of different forms of kidney diseases, including acute kidney injury (AKI) and chronic kidney diseases (CKD).488 PGC-1s have attracted increased attention in kidney diseases as outstanding regulators situated at the crossroads of mitochondrial energetics. Genetic study has illustrated that PGC-1α directs renal progenitor fate and is necessary for appropriate nephrogenesis in zebrafish.489 Of note, PGC-1α is abundantly present in the kidney, but PGC-1β is hardly expressed in the kidney and related research barely exists.1,16 In this section, the roles of PGC-1α in kidney diseases are emphasized.
PGC-1s in AKI
AKI, formerly termed acute renal failure, is a heterogeneous syndrome featuring by a sudden decrement in the glomerular filtration rate and the rapid loss of the excretory function.490 PGC-1α is reported to be downregulated in AKI induced by several factors, including ischemia, sepsis, and toxin.491–493
Kidney ischemia-reperfusion injury (IRI), universally occurring in renal transplantation, shock, trauma, and urologic and cardiovascular surgery, is a severe common clinical event leading to rapid kidney dysfunction and AKI.494 After 24 h of kidney IRI, the renal PGC-1α expression is downregulated and PGC-1α-/- mice exhibit worsened renal function, increased fat accumulation, and more severe tubular injury. The deeper investigation revealed that PGC-1α promotes NAD de novo synthesis from amino acids by upregulating related enzymes, whereas PGC-1α deficiency weakens the de novo pathway.491 In contrast, PGC-1α overexpression or activation following IRI facilitates the recovery of renal function and tubule homeostasis.495–498 Closely following this idea, Pan et al. recently found that PGC-1α overexpression enhances the interaction protein between mitochondria and ER and decreases the ER stress regulator hairy and enhancer of split 1, which blocks ER stress and apoptosis, thus protecting renal function during IR-induced AKI.216 Besides, FOXO1 inhibits PGC-1α transcription by competing with CREB for binding to transcriptional coactivators CREBBP/EP300. Conversely, FOXO1 inhibition prevents renal tubular epithelial cells apoptosis, ROS overproduction, and IR-induced downregulation of PGC-1α, then improves mitochondrial biogenesis, suggesting that FOXO1 inhibition prevents renal IRI via CREB/PGC-1α-mediated mitochondrial biogenesis.499 Brain and muscle ARNT-like 1 (BMAL1), as a pivotal regulator in circadian rhythm, also mediates mitochondrial homeostasis in renal IRI by activating the SIRT1/PGC-1α signal. BMAL1 overexpression significantly restrains apoptosis and oxidative stress, accompanied by the upregulated mRNA and protein levels of SIRT1, PGC-1α, NRF1, and TFAM, whereas SIRT1 inhibitor partially reverses the anti-apoptotic effect of BMAL1 overexpression, reflecting that BMAL1 mediates mitochondrial homeostasis through the SIRT1/PGC-1α axis in kidney IRI.500 In addition, some clinical drugs, including N-acetylcysteine, dexmedetomidine, eplerenone, and treprostinil, also exert positive anti-IRI effects on renal tissue by targeting PGC-1α.501–504
The kidney is one of the most common organs affected by sepsis and sepsis-associated acute kidney injury (sepsis-AKI) accounts for approximately half of AKI syndrome in ICU, significantly worsening patient prognosis.505 By kidney biopsies in patients who died of sepsis-AKI and control patients undergoing tumor nephrectomy, Slikke et al. found that the target genes of PGC-1α, such as TFAM, PINK1, and Parkin, are reduced in sepsis-AKI patients, which likely causes a reduction in mitochondrial mass.506 In the sepsis-AKI animal model, downregulated PGC-1α both at mRNA level and protein level are observed.492,493 Remarkably, lipopolysaccharide (LPS)-mediated suppression of PGC-1α reduces expression of downstream regulators of mitochondrial biogenesis, electron transport chain proteins, and renal cortical mtDNA content, finally disrupting mitochondrial homeostasis and resulting in renal dysfunction.493 Similarly, both in the LPS challenge and cecal ligation and perforation model, PGC-1α expression is proportionally suppressed with the degree of renal impairment. Meanwhile, PGC-1α expression and oxygen consumption decreases when exposed to TNF-α in tubular cells, whereas excessive PGC-1α reverses the latter effect.199 Together, these results provide strong evidence that the suppression of PGC-1α is a chief culprit event that affects functional impairment in sepsis-AKI.
In the setting of toxin-mediated AKI, the levels of PGC-1α and its target genes are also downregulated.507 In folic acid-induced AKI mice, the inflammatory cytokine TWEAK causes the reduction of PGC-1α expression and loss of MMP. TWEAK promotes histone H3 deacetylation at NF-κB-binding sites at the murine PGC-1α promoter in renal tubular cells and the activation of NF-κB, which impairs mitochondrial function.507 The same group further revealed that PGC-1α-/- mice manifest lower survival, more severe renal dysfunction, and an earlier decrement in mitochondrial mass than WT mice. Mechanically, PGC-1α deletion induces higher rates of tubular cell death, compensatory proliferation, expression of proinflammatory cytokines, NF-κB activation, and interstitial inflammatory cell infiltration.508 Conversely, in the cisplatin-induced AKI model, overexpression of PGC-1α or PGC-1α activator (ZLN005) treatment blocks cell apoptosis and mitochondrial dysfunction, finally alleviating kidney injury. Furthermore, ZLN005 treatment activates mitophagy, as manifested by increased expression of LC3-II and co-localization between LC3 and mitochondria, and the protective effects are abrogated in TFEB-knockdown cells, suggesting that PGC-1α activation improves mitochondrial dysfunction via TFEB-mediated autophagy.509 Additionally, the protective roles of ALDH2, aspirin, and liraglutide via attenuating mitochondrial dysfunction are reliant on PGC-1α-mediated biogenesis.510–512
PGC-1s in CKD
CKD is characterized by a reduced kidney filtration function, accompanied by nephron loss, inflammation, and extracellular matrix deposition.513 With a huge global burden and a prevalence of 10-14%, CKD is now considered a public health priority.514 The important roles of PGC-1α in CKD, especially diabetic kidney disease (DKD) and kidney fibrosis, have been noted.
DKD remains one of the fastest-growing causes of CKD and approximately 40% of diabetic patients develop DKD.515 Metabolomics analysis indicated that PGC‐1α expressions are downregulated in CKD patients, with a reduction in mitochondrial protein and mtDNA and impaired FAO.516,517 In high glucose (HG)-treated rat kidney mesangial cells, FOXO1 inhibition induced by HG downregulates PGC-1α expression, giving rise to mitochondrial dysfunction and ROS generation, while FOXO1 overexpression markedly increases PGC-1α, NRF-1, and Mfn2 expression, and decreases malondialdehyde production and proteinuria.518 In line with this, Guo and colleagues illuminated that hyperglycemia leads to the decrement of PGC-1α, which upregulates DRP1 expression, increases mitochondrial fragmentation, and damages network structure, but PGC-1α overexpression counteracts these alterations.519 These data suggested that PGC-1α may protect rats against DKD via the attenuation of mitochondrial dysfunction and ROS production. Moreover, the application of mesenchymal stem cells (MSCs) in the treatment of DKD has shown good prospects.520 By a coculture system consisting of MSCs and macrophages, it was found that MSCs-derived mitochondria are transferred into macrophages and this transfer stimulates PGC-1α-mediated mitochondrial biogenesis in parallel with the interaction between PGC-1α and TFEB in HG-induced macrophages, leading to the elevated lysosome-autophagy, ultimately ameliorating DKD.521 Similar results also exist in the streptozotocin-induced DKD rat model. When MSCs are injected into rats, podocyte injury and PINK1/Parkin-mediated mitophagy are ameliorated, which relies on the activation of the SIRT1-PGC-1α-TFAM pathway.522 In addition, some natural products, including resveratrol, berberine, purple rice husk, and formononetin, as well as clinical drugs such as rosiglitazone and rosiglitazone, exhibit protective effects in DKD by performing anti-oxidative effects, anti-apoptosis effects, and preventing mitochondrial dysfunction, in which PGC-1α is a principal hub mediator.517,523–528
Kidney fibrosis, characterized by excessive extracellular matrix deposition leading to scarring, is a key determinant of virtually all progressive CKD.529 Yang et al. identified PGC-1α as a negative regulator in EMT. Upregulated YY1 expression induced by HG promotes the formation of mTOR-YY1 heterodimer and the nuclear translocation of mTOR-YY1 inactivates PGC-1α by binding to the PGC-1α promoter, which further promotes mitochondrial dysfunction, leading to EMT and tubulointerstitial fibrosis in early DND.530 The transcription factor Twist1-induced downregulation of PGC-1α also facilitates kidney fibrosis by reducing FAO and increasing intracellular lipid droplet accumulation, mitochondrial dysfunction, and production of pro-fibrogenic factors.531 It is known that inflammation is the initiator and key link to ensuing fibrosis. In the kidney, PGC-1α inhibits the NLRP3 inflammasome to prevent kidney fibrosis. Mechanically, PGC-1α significantly mitigates the oligomerization of NLRP3 with the adapter protein ASC, the release of mtDNA from the mitochondria into the cytosol, and mitochondrial ROS and restores the expression of TNFAIP3 (a negative regulator), thus inhibiting NLRP3 inflammasome complex formation.209 In addition, tubule-specific overexpression of PGC-1α ameliorates Notch1-induced kidney injury, as manifested by the restoration of impaired mitochondrial morphology and FAO defect, and the reduction of apoptosis.87 The upregulation of PGC-1α by pharmacological approach also alleviates kidney fibrosis via maintaining mitochondrial homeostasis.532,533
In aggregate, the functional impacts of PGC‐1α in CKD have been conclusively demonstrated in preclinical studies, as PGC‐1α deficiency shows adverse effects, while genetic PGC‐1α overexpression or pharmacological PGC-1α upregulation is generally beneficial. However, excessive PGC-1α alters mitochondrial properties and induces podocyte proliferation and dedifferentiation, causing collapsing glomerulopathy.534 Therefore, controlling the exact levels of PGC-1α and establishing the optimal therapeutic window for PGC‐1α activation is significant to achieve clinical benefits.
PGC-1s in motor system diseases
Owing to the high expression of both PGC-1α and PGC-1β in skeletal muscle and the significance of continual supply of ATP in skeletal muscle contraction, it is not unexpected that PGC-1α and PGC-1β have been the research hotspot in skeletal muscle. Recently, the essential roles of PGC-1α and PGC-1β in bone homeostasis have gained considerable popularity and been well-established. For example, PGC-1α mediates osteoblastogenesis and PGC-1β modulates osteoclastogenesis,36,535–537 which orchestrates delicate balance between bone resorption and bone formation. Therefore, here we will focus on PGC-1α and PGC-1β in motor system diseases.
PGC-1s in osteoarthritis
Osteoarthritis, the most prevalent chronic joint disease, is a major source of pain, disability, and socioeconomic cost worldwide in accordance with the increased aging population and the epidemic of obesity.538 Notably, the upregulation of PGC-1α by activating the upstream molecule or coactivating the partners, remarkably reverses impaired mitochondrial biogenesis, oxidative stress, and inflammation in osteoarthritis.539–544 Nevertheless, classical drug therapy may be too late to help due to the relatively late diagnosis during the osteoarthritis process. Fortunately, emerging therapies targeting PGC-1α may possess great potential. For instance, mitochondrial transplantation can boost mitochondrial biogenesis in chondrocytes by activating PGC-1α signaling. It was found that the mitochondria of BMSCs could be ingested by rat chondrocytes via intra-articular injection and this mitochondrial transplantation successfully activates PGC-1α signaling, followed by suppressed inflammation, inhibited chondrocytes apoptosis, and improved mitochondrial biogenesis.545 More interestingly, zhou et al. conducted a cartilage-targeting dual-drug delivery nano platform (RB@MPMW) composed of rapamycin loaded into the mesopores and bilirubin loaded onto the shell of the metal organic-framework. RB@MPMW can continuously phosphorylate AMPK and further rescue mitochondrial energy metabolism of chondrocytes following IL-1β stimulation via activating the SIRT1-PGC-1α signaling pathway.546
PGC-1s in DMD
Duchenne muscular dystrophy (DMD), caused by the lack of functional dystrophin protein, is a lethal and progressive disease that leads to difficulties with movement and, eventually premature death.547 Amusingly, some gene programs associated with PGC-1α function, including mitochondrial OXPHOS, ROS detoxification, and Ca2+ signaling, are dysregulated in DMD,548–552 suggesting the feasible connection between PGC-1α and DMD. Importantly, PGC-1α stimulates a powerful program of neuromuscular junctions-linked gene expression both in myotubes and in vivo. Moderately upregulated PGC-1α in skeletal muscle improves fiber damage and fiber necrosis, and decreases serum creatine kinase levels, thereby exerting a beneficial effect in sedentary DMD mice.553 When PGC-1α is transferred into already declining muscle, the areas of immune cell infiltration and hypercontracted cells are decreased, and dystrophic muscle is rescued.554,555 A recent study indicated that PGC-1α overexpression increases TFEB nuclear localization and lysosome abundance and decreases the severity of DMD in dystrophin-deficient skeletal muscle.556
PGC-1s in sarcopenia
Sarcopenia, a geriatric disease characterized by a progressive loss of skeletal muscle mass and loss of muscle function, dramatically impinge on life quality and healthcare cost.557 Mitochondria usually undergo age-associated changes and their functions are impaired simultaneously, which enables mitochondria dysfunction to be one of the main attributors to sarcopenia progression.558 Liu et al. found that the senescence-accelerated mouse prone 8 exhibits typical features of sarcopenia at 40 weeks of age, but the decrement of genes involved in mitochondrial biogenesis (PGC-1α, NRF-1, TFAM, Ndufs8, and Cox5b) and mitochondrial dynamics fission (Mfn2 and Opa1) and autophagic flux are impaired from week 24, suggesting that early alterations of mitochondrial quality control and autophagic flux worsen muscle microenvironment prior to the onset of sarcopenia.559 However, PGC-1α overexpression attenuates these age-related increases in mitophagy markers and effectively ameliorates mitochondrial deficits, muscle and adipose tissue functionality, and systemic energy metabolism in aged mice.560,561 Genome-wide transcriptional changes analysis from genome-wide transcriptional changes in sarcopenia versus age-matched controls in muscle biopsies revealed that sarcopenia reproducibly manifests low PGC-1α/ERRα signaling, which may explain the global mitochondrial dysfunction including mitochondrial bioenergetic dysfunction, and downregulated OXPHOS and mitochondrial proteostasis.562 Notably, Ono et al. established a novel sequential drug screening system and identified an aminoindazole derivative, locamidazole, which can enhance locomotor function, and strengthen muscle and bone by inducing myocyte enhancer factors 2 c (MEF2c) and PGC-1α in a calcium signaling-dependent manner.537 Briefly, maintaining an optimal intracellular PGC-1α level and signaling activity contributes to protecting the muscle from sarcopenia.
PGC-1s in metabolic disorders
In recent decades, the prevalence and incidence of metabolic disorders, including T2DM, obesity, and metabolic dysfunction-associated steatotic liver disease (MASLD), have dramatically increased worldwide, imposing a staggering burden on whole society as well as individuals.563 Some key features of metabolic disorders cover impaired mitochondrial function, a decrement in glucose oxidation and FAO, and insulin resistance.564 Courtesy of the principal roles in energy metabolism and insulin sensitivity, PGC-1s may be considered as candidate factors in the etiology and therapeutics of metabolic disorders.
PGC-1s in T2DM
There is a growing prevalence of T2DM and its accompanied complications, including DCM, DKD, and diabetic neuropathy in the world.565 The pathogenesis is related to a combination of defects in insulin secretion by β-cells and impaired insulin sensitivity in insulin-responsive tissues, such as the liver, skeletal muscle, and adipose tissues.566 Over the past two decades, numerous evidence has shown that the expressions of PGC-1α and its downstream responsive genes, which are involved in mitochondrial biogenesis and OXPHOS, are downregulated in human and animal models with T2DM in skeletal muscle and adipose tissue.567–572 Conversely, the expression of PGC-1α in the liver are increased in diabetic mice.573 Thus, it is not difficult to speculate that the roles of PGC-1s in T2DM depend on the tissue. Importantly, evidence from tissue-specific transgenic or knockout animal models of PGC-1s have supported this notion.
As the principal tissue for the majority of insulin-stimulated whole-body glucose disposal, skeletal muscle is a primary controller of whole-body glucose homeostasis and insulin sensitivity.574 As mentioned above, the electro-transfection or overexpression of PGC-1α upregulates GLUT4 expression and glucose uptake in skeletal muscle.226,227 Meanwhile, impaired glucose disposal in skeletal muscle leads to insulin resistance and accelerates the development of T2DM.575 Notably, PGC-1α hold precise control for glucose disposal by involving in multiple glucose metabolic processes.228,229 For example, PGC-1α increases muscle glycogen stores by suppressing glycolytic flux, and downregulating the expression of glycogen phosphorylase and phosphorylase kinase α.228 In adipose tissue, reduced expression of PGC-1 and insulin-signaling molecules is associated with adipose tissue dysfunction, which further impairs the systemic insulin response in the insulin-resistant subjects.570 These findings emphasize the potential of PGC-1α activation in the treatment of T2DM.
The liver is an important organ in driving gluconeogenesis. In a diabetic model, overexpression of PGC-1α in the liver causes hepatic insulin resistance, manifested by higher glucose production and diminished suppression of gluconeogenesis by insulin.573 PCAF is an acetyltransferase of PGC-1α and liver-specific knockdown of PCAF increases PGC-1α activity, which further upregulates blood glucose and hepatic glucose output.576 Conversely, selectively inhibiting the gluconeogenic activity of PGC-1α in the liver using SR-18292 (a small molecule) improves hepatic insulin sensitivity and glucose homeostasis in diabetic mice.577 Similarly, ZLN005 reduces PGC-1α mRNA levels and gluconeogenesis genes in the liver, while increasing PGC-1α and improving glucose utilization and FAO in skeletal muscle.19 In addition, the spexin peptide can repress hepatic gluconeogenesis in both HFD-induced rats and insulin-resistant cells to ameliorate insulin resistance, which also relies on the FOXO1/PGC-1α pathway.224 Pancreatic β cells are mainly responsible for synthesizing and secreting insulin. Similar to the liver, overexpressing PGC-1α in isolated rat islets suppresses membrane polarization and induces G6P, thereby inhibiting insulin secretion.578 In addition, inducible β-cell PGC-1α overexpression in fetal life leads to decreased β-cell mass, and β-cell hypotrophy, decreased insulin secretion, and damaged glucose tolerance.579
Apart from the diabetic complications discussed above, such as DCM and DKD, PGC-1α is involved in the development of other DM-related organ damage, such as diabetic neuropathy and vascular dysfunction. The most prevalent complication is neuropathy and at least 50% of individuals with diabetes develop diabetic neuropathy over time.580 Diabetic mice are usually accompanied by peripheral neuropathy, decreased mitochondria and mitochondrial DNA, and increased protein oxidation. Notably, the loss of PGC-1α further aggravates this phenotype and is associated with mitochondria degeneration and increased oxidative stress, while overexpression of PGC-1α in neurons prevents oxidative injury caused by high glucose. These supported the idea that knockout of PGC-1α increases susceptibility to diabetes-induced neuropathy.581 In diabetes, the PGC-1α expression in endothelial cells are upregulated. Endothelial PGC-1α effectively inhibits endothelial migration in cell culture and angiogenesis in vivo, leads to aberrant re-endothelialization after carotid injury, blunts wound healing, and reduces blood flow recovery after ischemia. Further mechanism exploration shown that PGC-1α induces Notch signaling, blocks activation of Rac/Akt/eNOS signaling, and renders endothelial cells unresponsive to angiogenic factors, finally contributing to vascular dysfunction in diabetes.582 In addition, T2DM disrupts SIRT1/PGC-1α/SIRT3 pathway in the epididymal, which causes a decline of the antioxidant defenses and an increased oxidative damage in that tissue, ultimately leading to impaired male reproductive function.583
PGC-1s in obesity
Currently, obesity is increasing in an alarming rate (tripling over the past four decades) worldwide,584 and causes higher risks of some diseases, including T2DM, MASLD, and cardiovascular diseases.585 Continuous expansion of white adipose tissue (WAT) and subsequent ectopic accumulation throughout the body is the chief culprit of obesity, while BAT consumes glucose and triglycerides, thus generating heat.586 As described above, PGC-1 was initially cloned from a brown fat cDNA library and shown to drive adaptive thermogenesis in BAT.1 In the adipose tissue of obese subjects or mice models, mitochondrial biogenesis regulator PGC-1α, OXPHOS protein levels of complexes I and III, and oxidative metabolic pathways are also reduced.572,587–589 Recently, emerging studies have revealed the roles of PGC-1s in adipose tissue.
Obese mice exhibits a marked reduction of PGC-1α, which is accompanied with adipocyte hypertrophy, fibrosis, and decreased mitochondrial respiration.590 Kleiner et al. investigated the effects of adipose-specific PGC-1α deficiency on systemic glucose homeostasis. The results showed that when mice with PGC-1α deficiency in WAT are exposed to HFD, they develop insulin resistance and experience decreased suppression of hepatic glucose output.225 On the contrary, adipose-specific overexpression of PGC-1α improves mitochondrial biogenesis and respiration, and decreases fasting glucose, blood pressure, and fibrosis. Meanwhile, PGC-1α upregulates the expression of processes associated with the browning of fat tissue, including UCP1, FGF21, and p-AMPK signaling, with a reduction in inflammatory adipokines, NOV/CCN3 expression, and TGFβ. These findings highlight the beneficial impact of adipose-PGC-1α on metabolic disturbances.590
As a downstream effector of some transcription factors, PGC-1α mediates their regulatory roles in obesity. For example, Foxj3 overexpression in primary brown adipocytes enhances energy expenditure and improves systemic metabolism on either a chow diet or an HFD. Mechanistically, cold-inducible Foxj3 stimulates the expression of PGC-1α and UCP1, subsequently promoting energy expenditure.591 The transcription factor GATA3 mitigates obesity by activating thermogenesis and improving energy expenditure through the upregulation of UCP-1 expression via its interaction with PGC-1α.592 TFEB is a basic helix-loop-helix transcription factor. Adipocyte-specific TFEB overexpression protect mice from diet-induced obesity, insulin resistance, and metabolic sequelae. Importantly, adipocyte-specific PGC-1α deficiency also markedly blocks the effects of TFEB overexpression on the induction of browning genes in WAT, as well as diet-induced weight gain and adiposity, suggesting that these metabolic phenotypes of TFEB overexpression are PGC-1α-dependent.593 Furthermore, cardiotrophin-like cytokine factor 1 (CLCF1) is a negative regulator of PGC-1α and PGC-1β. Adipocyte-specific CLCF1 transgenic mice develops severe cold intolerance and metabolic dysfunction, partially due to the inhibition of PGC-1α and PGC-1β, which results in impaired mitochondrial biogenesis. This indicates that targeting this pathway restores brown fat activity and systemic metabolic homeostasis in obesity.594 Besides, IL-27-IL-27Rα signaling has been found to improve thermogenesis and insulin resistance and protect against obesity. Further investigation showed that IL-27 directly targets adipocytes to elicit the activation of p38 MAPK, thereby enhancing the activation of ATF2 and the expression of PGC-1α and UCP1.595
Besides, Kamei et al. found that total energy expenditures increase by up to 1.3 times when the expression of PGC-1β in skeletal muscle is slightly augmented. Consequently, less fat is accumulated and stored.128 In 3T3-L1 adipocytes, overexpression of PGC-1β improves insulin sensitivity and mitochondrial function.176 In contrast to this, adipose-specific ablation of PGC-1β impairs thermogenesis and reduces the number of contacts between mitochondria and lipid droplets.596 These findings demonstrate that PGC-1β contributes to the control of energy balance and provide a potential approach for developing novel anti-obesity drugs.
PGC-1s in MAFLD
MAFLD affects up to a third of the global population in parallel with a growing epidemic of obesity and T2DM.597 HFD can lead to a state of nonalcoholic fatty liver disease (NAFLD), accompanied by the decreased expression of PGC‑1α and subsequent hepatic inflammation. PGC-1α downregulation promotes phosphorylation of IκBα and subsequent increase in nuclear translocation of p65 NF-κB, ultimately increasing the expression of proinflammatory cytokines.123 P2Y2R is a subtype of purinergic P2 receptor. P2Y2R deficiency effectively improves insulin resistance and attenuates hepatic lipid accumulation and injury by enhancing FAO through activation of AMPK signaling and PGC-1α pathway.598 In addition, PRMT1, the major protein arginine methyltransferase in mammals, is involved in the transcription, splicing, RNA biology, the DNA damage response, and cell metabolism.599 Previous vitro experimental confirmed that PRMT1 promotes hepatic lipogenesis via the TXNIP/PRMT1/PGC-1α pathway.600 However, a recent vivo study found that overexpression of PRMT1 in HFD-fed mice alleviates hepatic steatosis by enhancing PGC-1α-mediated FAO via recruitment of HNF4α to the promoter of PGC-1α.601 Although the observed results are contradictory, partially due to substantial differences between in vitro and in vivo experiments, all these highlight the important regulatory roles of PGC-1α in MAFLD. Further comprehensive and in-depth exploration will be beneficial in manipulating PGC-1α as a clinical treatment of MAFLD.
Like PGC-1α, PGC-1β plays a dual role in hepatic lipid metabolism. Selective activation of PGC-1β within hepatocytes can prevent liver lipid overload and fibrosis by inducing mitochondrial OXPHOS, FAO and citrate cycle.239 The forkhead box protein subfamily member FOXA2 regulates glucolipid metabolism and is closely correlated with hepatic steatosis and NAFLD.602 Notably, PGC-1β can coactivate with FOXA2 and modulate hepatic lipid homeostasis. Adenoviral expression of FOXA2 and PGC-1β in the livers of ob/ob mice decreases hepatic triacylglycerols content, increases plasma triacylglycerols concentrations, and promotes apolipoprotein B-containing very-low-density lipoprotein secretion.603 However, several studies have suggested that PGC-1β coordinates hepatic lipogenic capacity via interactions with multiple lipogenic transcription factors. Nagai et al. demonstrated that PGC-1β knockdown decreases hepatic de novo lipogenesis, hepatic triglyceride synthesis, and hepatic and peripheral insulin resistance induced by fructose through reducing the expression of sterol regulatory element-binding protein (SREBP)-1 and downstream lipogenic genes in liver.231 Furthermore, retinol binding protein 4 (RBP4) induces SREBP-1 activation and consequently accelerates hepatic lipogenesis and plasma triglyceride, but this phenomenon is not observed in PGC-1β knockout mice.604 ChREBP is a glucose responsive transcription factor. PGC-1β-mediated coactivation of ChREBP induces genes encoding glycolytic and lipogenic enzymes response to hyperglycemia, whereas liver-specific PGC-1β deficiency impairs the lipogenic response to high glucose conditions.237
Application of Pgc-1s
Application of PGC-1s in cancer
The diagnostic and prognostic value of PGC-1s in cancer in clinical studies
In certain types of cancer, especially those affecting the female reproductive system, alterations in the expression of PGC-1s have manifested significant diagnostic and prognostic value. In OC, the expression of PGC-1α and ERRα exhibits significantly higher in cancer tissues compared to noncancerous tissues, and high expression of PGC-1α is remarkably associated with tumor differentiation. The analysis that combined high PGC-1α and ERRα expression predicts a tendency towards poor cancer-specific survival.605 In EC, the expression of PGC-1α and ERRα is higher in highly invasive EC tissues than in less invasive EC and significantly higher than in normal tissues. A single-factor logistic regression analysis confirmed that PGC-1α and ERRα may serve as novel biomarkers for predicting the risk of advanced myometrial invasion.606 Similarly, increased levels of PGC-1α in BC patients are correlated with more aggressive cancer characteristics, as well as poorer disease-free survival and overall survival in comparison to patients with lower plasma levels.607 Additionally, in CRC, there is a significant correlation between PGC-1α expression and nodal metastasis. The PGC-1α-positive group has reduced overall survival compared to the PGC-1α-negative group, suggesting that PGC-1α represents a biomarker for nodal metastasis and poor prognosis.279 In contrast to the above conclusions, high levels of PGC-1α in non-small cell lung cancer are indicative of a positive prognosis. This is supported by the fact that patients with elevated levels of PGC-1α has a median overall survival higher over 24 months, whereas those with low PGC-1α expression only survive for a median of 15.4 months.608
Pre-clinical studies of PGC-1s in cancer treatment
Natural products or molecules by targeting PGC-1s in cancer
Currently, no specific drugs targeting PGC-1s in cancer are commercially available in clinics. In pre-clinical studies, the compound that exerts its protective effect by activating PGC-1s or inhibiting PGC-1s are both present.
SR18292, a PGC-1α inhibitor, leads to dysfunction in OXPHOS metabolism, energy exhaustion, and oxidative damage, thus impairing the proliferation and survival of multiple myeloma cells.609 Metformin, a first-line drug treatment for T2DM, also increases H2O2-induced cancer cell death. It downregulates Nrf2 expression by suppressing PGC-1α-mediated PPARγ transcriptional activity, which enhances the susceptibility of WT p53 cancer cells to oxidative stress and therapeutic agents.610 Furthermore, the herbal medicine Paris polyphylla has been confirmed to inhibit OC. It remarkably decreases the level of PGC-1α, which in turn markedly suppresses the elevated expression of vimentin and recovers the expression of E-cadherin in HG-induced OVCAR-3 cells.611 Additionally, isoliquiritigenin, a common herb used in traditional Chinese medicine, inhibits the expression of PGC-1α at protein level and enhances ROS accumulation in gastric cancer cells, but PGC-1α overexpression partly reverses the inhibition of ISL on cell viability.612 On the other hand, bouchardatine (an alkaloid derived from B. Neurococca) suppresses cancers via PGC-1α activation. It effectively induces a metabolic reprogramming towards aerobic metabolism by upregulating UCP2 through PGC-1α enrichment in its promoter, finally blunting rectal cancer cell proliferation.613
Targeting PGC-1s combination with antitumor immunity
T cell immunotherapy have provided new therapeutic dawn for a wide range of cancer patients, but T cell exhaustion may also represent an inherent impediment in exerting long-lived antitumor effects.614 Mitochondria have taken the spotlight as important regulators at different stages of T cell development, while mitochondrial dysfunction is an upstream driver of T cell exhaustion.615 Recently, numerous studies have highlighted the potential of targeting PGC-1α in combination with antitumor immunity owing to the predominant roles of PGC-1α in mitochondrial function. PGC-1α activation induced by bezafibrate coactivates NRFs and PPARs, further promoting a series of transcription factors, which enhances FAO and OXPHOS, and mitochondrial expansion, thereby facilitating cytotoxic T lymphocytes (CTL) activation and proliferation.616 Then, the same group further found that bezafibrate with PD-1 blockade induces mitochondrial biogenesis and FAO in CD8 + T cells and maintains the number of functional CTLs, which enhances antitumor immunity during PD-1 blockade.617 The evidence from another team in lung cancer also supported the similar conclusion.618
The enforced expression of PGC-1α promotes CD8 T cell persistence, memory formation, and antigen recall potential, and maintains more robust recall responses to bacterial infection or peptide vaccination. PGC-1α-overexpressing CD8 T cells also has remarkably improved antitumor efficacy.619,620 PGC-1α also links epigenetic modification and anti-tumor immunity. Ketogenesis-derived β-hydroxybutyrate, present in CD8+ memory T cells, upregulates Pck1 expression by epigenetically modifying Lys 9 of histone H3 (H3K9) of FOXO1 and PGC-1α, which directs the carbon flow along the gluconeogenic pathway to glycogen and the pentose phosphate pathway, thus promoting CD8 + T-cell memory development.621 Besides, Malinee et al. designed a DNA-based epigenetic activator with tri-arginine vector called EnPGC-1, which can stimulate the targeted induction of the PGC-1α/β. Importantly, EnPGC-1 enhances mitochondrial activation, energy metabolism, proliferation of CD8 + T cells, and OXPHOS, thereby improving the longevity and effector functions of killer T cells and augments the efficacy of PD-1 blockade in combination.622 Interestingly, an engineered version of PGC-1α containing a point mutation at S571 (PGC-1αS571A) has been developed by Lontos and colleagues. PGC-1αS571A transduction endows CAR-T cells potent mitochondrial reprogramming, which drives more effector-like programs and a more long-lived memory state. Therefore, PGC-1αS571A transduced CAR-T cells treatment provides stronger antitumor immunity, and longer survival for all mice.623
Taken together, these explorations suggest that targeting PGC-1α combination with antitumor immunity can effectively improve the therapeutic efficacy, success in future clinical trials may benefit cancer patients, especially those who are unresponsive to T cell-based monotherapy.
Application of PGC-1s in non-cancer diseases
The diagnostic and prognostic value of PGC-1s in non-cancer diseases in clinical studies
The altered expression of PGC-1s in various diseases have been described in previous parts. In this section, we focus on examining the connection between PGC-1s gene polymorphism and susceptibility to diseases.
Neurological disorders
It has been demonstrated that the coding variant rs3736265 and rs6821591 in PPARGC1A has a significant effect on the age of onset in the population carrying the HD mutation.624,625 Moreover, Che et al. discovered the influence of two other single nucleotide polymorphisms (SNP) of PGC-1α in HD. While the minor allele of SNP rs7665116 (g.38570 C), located in the transcribed gene region, is linked to a delay in disease onset, the minor allele of SNP rs2970870 (g.-1437C) in the promoter region contributes to an earlier onset of HD in its homozygous state.626 Interestingly, no relation between PGC-1α Gly482Ser polymorphism and oxidative stress biomarker levels is detected in ALS patients under resting conditions. However, during exercise performance, significantly higher lactate levels and greater protein oxidative products are found in AA (Ser482Ser) ALS patients compared to GG (Gly482Gly) and GA (Gly482Ser).627
Metabolic disorders
The association between PPARGC1A polymorphism and T2DM have been extensively investigated, mainly PPARGC1A Gly482Ser. At first, Kunej et al. found that the AA genotype of the Gly482Ser polymorphism is related to 1.9-times increased risk of T2DM and is considered as a risk factor for the development of T2DM in Caucasians.628 The PGC-1α Gly482Ser allele can also predict the conversion from impaired glucose tolerance to T2DM.629 Then, over two decades, the researchers conducted a large number of studies. However, conflicting results have also emerged from different studies, which largely depends on population sample sizes, environmental context (area, nation and so on), the tissue-specific functions of the allele, and perhaps even the stage of disease progression.629–638
Additionally, in NAFLD, the PPARGC1A rs8192678 risk A allele is associated with an increased risk, even after control for BMI and other confounding factors.639 Nevertheless, the Gly482Ser polymorphism of the PGC-1α gene is not associated with the metabolic syndrome in Danish Caucasian subjects.640 Interestingly, Huang et al. utilized engineered allele substitution at PPARGC1A rs8192678 to obtain homozygous AA, GG and heterozygous G/A isogenic cell populations. It was shown that the C allele causes reduced levels of PPARGC1A mRNA and PGC-1α protein, along with disrupted dynamics of PGC-1α turnover and activity, which subsequently impacts cellular differentiation and mitochondrial function.641 Further studies on the underlying mechanisms in the future may potentially offer novel insights into the discrepancies observed across clinical studies.
Pre-clinical studies of PGC-1s in non-cancer diseases treatment
Medical treatment
The medical treatments targeting PGC-1s, mainly PGC-1α, have exhibited immense potential in various disease models in preclinical studies. Since a comprehensive presentation of all is too verbose, we will concentrate on a couple of natural products, such as resveratrol,642–649 curcumin,650–654 berberine,517,655–660 quercetin,661–669 or clinical drugs, which have been extensively investigated in different pathological models. Other representative compounds, including astragaloside IV,670–672 baicalin,673–676 dihydromyricetin,676–681 isoliquiritigenin,682,683 astragalus polysaccharide,684,685 dexmedetomidine,686–689 will be summarized in Table 1.
Table 1.
A summary of protective effects of natural or synthesized compounds targeting PGC-1s-related pathway in a variety of diseases
| Compounds | Models | Pathway/Targets | Effects | Refs |
|---|---|---|---|---|
| Resveratrol | Contrast-induced nephropathy | SIRT1/PGC-1α/FOXO1 | Reduces oxidative stress, inflammatory cell infiltration, and apoptosis | 642 |
| Hyperoxia lung injury | SIRT1/PGC-1α | Upregulates citrate synthase and TFAM expression | 643 | |
| Diabetic cardiomyopathy | SIRT1/PGC-1α | Ameliorates mitochondrial dysfunction | 644,645 | |
| Myocardial IRI | SIRT1/SIRT3-Mfn2-Parkin-PGC-1α | Regulates the balance of mitochondria fission-fusion, autophagic flux, and mitochondrial biosynthesis | 646 | |
| HG-induced kidney injury | SIRT1/PGC-1α | Inhibits oxidative stress and mitochondrial apoptosis pathway and ameliorates mitochondrial function | 647 | |
| HG-treated retinal | AMPK/SIRT1/PGC-1α | Inhibits ROS-induced apoptosis | 648 | |
| Hypoxia-treated OC cell | SIRT1/PGC-1α | Recovers SIRT1 and mtDNA expression and antagonizes CoCl2-induced VEGF production | 649 | |
| Neuronal cell injury | PGC-1α | Attenuates autophagy, the release of inflammatory cytokines and ROS generation, and enhances M2 microglial polarization and mitochondrial biogenesis | 690–692 | |
| Curcumin | Liver fibrogenesis | AMPK/PGC-1α | Inhibits collagenα1 and HSCs activation | 650 |
| Isoniazid-induced hepatotoxicity | SIRT1/PGC-1α/NRF1 | Reduces necrosis, oxidative stress, and inflammation | 651 | |
| Depression | PGC-1α/FNDC5/BDNF | Promotes neurocyte proliferation and suppresses neuronal apoptosis | 652 | |
| Tissue repair | PGC-1α/SIRT3/HIF-1α | Inhibits mitochondrial cytochrome c release and apoptosis | 653 | |
| Cisplatin-induced kidney injury | PGC-1α | Improves mitochondria biogenesis and prevents renal fibrosis and apoptosis | 654 | |
| Berberine | DKD | PGC-1α | Counteracts lipid accumulation, ROS production, mitochondrial dysfunction, and deficient FAO | 517 |
| Diabetic nephropathy | C/EBPβ/PGC-1α | Regulates mitochondrial energy metabolism, and inhibits ROS production and apoptosis | 655 | |
| Diabetic neuropathy | PGC-1α | Attenuates mitochondrial deficits and redox imbalance | 656 | |
| Fatty liver | PGC-1α | Improves mitochondrial respiratory chain function and insulin signaling | 657 | |
| Metabolic disorders | AMPK/PGC-1α | Promotes the mitochondrial biogenesis and FAO, and prevents excessive lipid accumulation | 658 | |
| Aging | AMPK/SIRT1/PGC-1α | Ameliorates aging-related reductions in cognitive ability and muscular function | 659 | |
| Alzheimer’s disease | GSK3β/PGC-1α | Inhibits tau hyperphosphorylation and neuroinflammation | 660 | |
| Quercetin | Traumatic brain injury | PGC-1α | Inhibits neuronal apoptosis and ameliorates mitochondrial lesions | 661 |
| Hypobaric hypoxia-induced memory impairment | SIRT1/PGC-1α | Reduces hippocampus mitochondrial and synaptic lesions | 662 | |
| H2O2-induced neuronal damage | SIRT1/PGC-1α | Triggers mitochondrial biogenesis and reduces oxidative stress damage | 663 | |
| NaIO3-induced retinal damage | Nrf2/PGC-1α/SIRT1 | Reverses oxidative stress and ROS production | 664 | |
| Myocardial IRI | SIRT1/PGC-1α | Inhibits cardiomyocyte apoptosis | 665 | |
| Aluminium-induced oxidative stress | PGC-1α | Inhibits oxidative stress and promotes mitochondrial biogenesis | 666 | |
| LPS-induced oxidative damage | SIRT1/PGC-1α | Upregulates the mitochondrial membrane potential, and reverse the mitochondrial morphology damage | 667 | |
| Alcoholic liver disease | PGC-1α | Downregulates redox status, lipid droplets, restores damaged mitochondrial membrane potential, and repairs mtDNA damage | 668 | |
| Vincristine-induced liver injury | Nrf2/HO-1, NF-kB/STAT3, SIRT1/PGC-1α | Attenuates oxidative stress, apoptosis, and autophagy | 669 | |
| Astragaloside IV | Peritoneal fibrosis | PGC-1α | Enhances mitochondrial synthesis and reduces apoptosis | 670 |
| Metabolism disorder | AMPK/PGC-1α | Enhances energy metabolism and inhibits apoptosis | 671 | |
| Isoproterenol-induced cardiac hypertrophy | NF-κB/PGC-1α | Regulates energy biosynthesis | 672 | |
| Baicalin | Depression | AMPK/PGC-1α | Improves mitophagy level and mitochondrial function | 673,674 |
| Insulin resistance | p38 MAPK/PGC-1α | Decreases body weight, HOMA-IR, and alleviates HFD-induced glucose intolerance, hyperglycemia, and insulin resistance | 675 | |
| Pulmonary hypertension | PGC-1α | Ameliorates angiogenesis | 676 | |
| Dihydromyricetin | Diet-induced obesity | IRF4/PGC-1α | Reduces body weight, decreases WAT mass, improves glucose and lipid metabolic disorders, and ameliorates hepatic steatosis | 677 |
| Gentamicin-induced ototoxicity | PGC-1α/SIRT3 | Protects cells from apoptotic death by inhibiting ROS accumulation | 678 | |
| Dexamethasone-induced muscle atrophy | PGC-1α | Stimulates mitochondrial biogenesis and promotes mitochondrial fusion, rescues the reduced mtDNA content, improves mitochondrial morphology | 679 | |
| Type 2 diabetes | AMPK/PGC-1α/SIRT3 | Activates insulin signaling and increases glucose uptake in skeletal muscle | 680 | |
| Alcoholic liver disease | AMPK/SIRT1/PGC-1α | Increases TFAM expression, hepatic ATP concentrations, and induces mitochondrial expression of respiratory complex III and V | 681 | |
| Isoliquiritigenin | LPS/D-GalN-induced acute liver failure | PGC-1α/Nrf2 | Improves the ability of anti-oxidative stress, alleviates inflammatory reaction and apoptosis | 205 |
| Alcoholic liver injury | miR-23a-3p/PGC-1α | Promotes fatty acid metabolism and inhibits the ROS | 682 | |
| Nonalcoholic fatty liver disease | miR-138-5p/PGC-1α | Promotes lipid metabolism and inhibits inflammatory response | 683 | |
| Astragalus polysaccharide | Cardiac hypertrophy | TNF-α/PGC-1α | Improves the cardiac hemodynamics | 684 |
| Insulin resistance | SIRT1/PGC-1α/PPARα | Suppresses abnormal glycolipid metabolism and insulin resistance | 685 | |
| Dexmedetomidine | Acute kidney injury | PGC-1α/STAT1/IRF-1 | Inhibits mitochondrial damage and inflammation | 502 |
| Traumatic brain injury | PGC-1α | Relieves encephala edema and neuron cell apoptosis and increases behavioral function | 686 | |
| Intracerebral hemorrhage | PGC-1α | Increases GPX and SOD levels and reduces MDA and nitric oxide levels | 687 | |
| Doxorubicin-cardiotoxicity | PGC-1α | Attenuates mitochondrial dysfunction, oxidative stress, and apoptosis | 688 | |
| OGD/R | PPARδ-AMPK-PGC-1α | Enhances the cell viability and decreases ROS production | 689 | |
| Melatonin | OGD/R; Myocardial IRI | PGC‑1α/Nrf2; AMPK/PGC1α | Represses oxidative stress and inflammation | 694,695 |
| Cardiac hypertrophy | PGC-1α/MICU1 | Ameliorates ROS generation and promotes mitochondrial function | 696 | |
| Ischemia | PGC-1α | Promotes OXPHOS and angiogenic ability of MSCs | 697 | |
| Kidney injury | AMPK/SIRT1/PGC-1α | Relieves oxidative stress, mitochondrial dysfunction, and apoptosis | 698 | |
| Diabetic myocardial IRI | PGC-1α | Improves mitochondrial quality control, alleviates diabetic cardiomyopathy, and reduces myocardial vulnerability to IRI | 166,699,700 | |
| Chromium-induced lung injury | SIRT1/PGC-1α/Nrf2 | Reduces oxidative stress and inflammatory mediators and inhibits cell apoptosis | 701 | |
| Rotenone-induced mitochondrial deficiency | SIRT1/PGC-1α | Abrogates mitochondrial dysfunction, ATP deficiency, oxidative stress, and apoptosis | 702 | |
| Cadmium-induced kidney injury | SIRT1/PGC-1α | Attenuates Drp1- and Fis1-mediated mitochondrial fission and mitochondrial oxidative stress | 703 | |
| Bisphenol A-induced colon injury | SIRT1/PGC-1α | Restores the mitochondrial dynamic balance and activates the Nrf2 antioxidant axis | 704 | |
| Metformin | High-glucose environment | AMPK/SIRT1/PGC-1α | Promotes cell proliferation, enhance GSIS, and suppresses apoptosis | 707 |
| p53 cancer cells | SIRT1/ PGC-1α/Nrf2 | Increases the susceptibility of p53 cancer cells to oxidative stress and TRAIL-induced apoptosis | 610 |
Resveratrol
Both preclinical experiments and clinical trials of resveratrol achieved tremendous benefits in a variety of human diseases, such as diabetes, cardiovascular diseases, neurodegeneration, and cancers, in which PGC-1α is a potential target.642–645 In terms of cardiovascular disease, resveratrol reestablishes the balance of mitochondria fission-fusion and regulates autophagic flux and mitochondrial biosynthesis through the SIRT1/SIRT3-Mfn2-Parkin-PGC-1α pathway in myocardial IRI.646 Asymmetric dimethylarginine and HFD promotes PGC-1α acetylation and results in DM, whereases resveratrol treatment remarkably reverses altered PGC-1α expression and acetylation in the myocardium, thus ameliorating cardiac and mitochondrial dysfunction.644,645 In CNS, resveratrol exerts neuroprotective effects against neuronal cell injury via attenuating autophagy, suppressing the release of inflammatory cytokines and ROS generation, and enhancing M2 microglial polarization and mitochondrial biogenesis.436,690–692 Under HG induced-kidney, SIRT1 and PGC-1α are downregulated, which exacerbates oxidative stress, activates mitochondrial apoptosis pathway, and impairs mitochondrial function, while resveratrol can partially offset these phenomena through the SIRT1/PGC-1a axis.647 In addition, resveratrol can also trigger the AMPK/ SIRT1/PGC-1α pathway to inhibit ROS-induced apoptosis in HG-treated retinal capillary endothelial cells.648 During hyperoxia, the activation of the SIRT1/PGC-1α signaling pathway by resveratrol attenuates lung injury and VEGF induction.649 Briefly, resveratrol, as a classical agonist of SIRT1, combats oxidative stress, inflammation, apoptosis, and mitochondrial dysfunction by activating the SIRT1/PGC-1α pathway, eventually providing protection against various diseases.
Curcumin
Curcumin, a crucial polyphenol present in Curcuma longa L. rhizome, exemplifies a promising traditional medicinal agent. Recent studies have revealed anti-apoptotic, anti-oxidative, and antidepressant properties of curcumin that arise from its modulation of PGC-1α. In the liver, curcumin activates AMPK and increases PGC-1α expression, then inhibiting collagenα1 and hepatic stellate cells (HSCs) activation, thus effectively preventing liver fibrogenesis.650 Severe hepatotoxicity greatly limits the application of isoniazid, a first-line drug in tuberculosis. Li et al. found that curcumin alleviates isoniazid-induced hepatotoxicity by upregulating the SIRT1/PGC-1α/NRF1 pathway.651 In chronic unpredictable mild stress-induced depression-like behavior, curcumin supplementation promotes neurocyte proliferation and inhibits neuronal apoptosis, while PGC-1α inhibitor SR18292 reverses the beneficial effects of curcumin on depressed rats.652 Of note, curcumin combined with other treatment methods shows tremendous treatment effects. For instance, curcumin combined with hypoxic preconditioning obviously promotes cell survival, improves mitochondrial function in BMSCs, and inhibits mitochondrial cytochrome c release as well as consequent apoptosis signal. However, PGC-1α RNAi simulates mitochondrial superoxide and H2O2 production in hypoxia.653 Co-treatment of curcumin with cisplatin promotes apoptosis and activates endothelin-1 clearance in the SKOV3 cell (Human OC cell line) and OC rat model, thus preventing renal fibrosis. These shed light on curcumin as a therapeutic adjuvant in the clinical setting.654
Berberine
Berberine is a representative isoquinoline alkaloid as well as an eminent component of traditional Chinese medicine for more than 2000 years.693 Berberine has the ability to suppress many diabetic complications.517,655,656 In db/db mice, berberine treatment inhibits lipid disorder-induced podocyte damage and development of DKD by counteracting lipid accumulation, ROS production, mitochondrial dysfunction, and deficient FAO, in which PGC-1α-mediated mitochondrial bioenergetics perform a key role.517 In neuronal cells, berberine treatment facilitates PGC-1α-mediated mitochondrial biogenesis and redox imbalance, thereby inhibiting diabetic neuropathy.656 Moreover, berberine affects the lipid deposition of skeletal muscle and liver.657,658 Mechanically, berberine activates the AMPK/PGC-1α pathway, thus promoting mitochondrial biogenesis and improving FAO, eventually preventing excessive lipid accumulation.658 Berberine also ameliorates aging-related reductions in cognitive ability and muscular function, which benefits from the activation of the AMPK/SIRT1/PGC-1α pathway.659 Meanwhile, it represses tau hyperphosphorylation and neuroinflammation, which is attributed to the regulation of the GSK3β/PGC-1α signaling pathway in APP/PS1 mice.660
Quercetin
Pre-clinical experiments of quercetin revealed their therapeutic efficacy in T2DM, AD, liver injury, and cardiac diseases. In neuronal cells, quercetin remarkably inhibits neuronal apoptosis and ROS generation, reestablishes mitochondrial biogenesis and dynamics, and ameliorates mitochondrial function by activating PGC-1α-related pathway.661–663 In the ARPE19 cells, NaIO3 exposure changes the retinal structure and suppresses pupil constriction, while quercetin treatment inhibits the generation of mitochondrial ROS, which is dependent on increased levels of deacetyl-SOD2 through the Nrf2-PGC-1α-SIRT1 signaling pathway.664 During myocardial IRI, quercetin can also mitigate apoptosis via SIRT1/PGC-1α signaling.665 In other injury models induced by LPS, aluminium, ethanol, or vincristine, quercetin treatment alleviates oxidative stress, apoptosis, autophagy, and mitochondrial homeostasis, accompanied by increased levels of PGC-1α.666–669 In summary, quercetin possesses powerful organ protective functions by targeting PGC-1α and may represent a therapeutic strategy.
Melatonin
Melatonin, the primary circadian output signal from the brain, is uncommonly effective in anti-oxidative stress, anti-inflammatory, anti-apoptosis, and anti-fibrosis, thus offering protection against a wide variety of diseases.694–698 For instance, in the OGD/R or myocardial IRI model, melatonin plays protective roles via the inhibition of oxidative stress and inflammation by regulating the PGC‑1α/Nrf2 and PGC‑1α/TNF‑α signaling pathways.694,695 Furthermore, in the setting of diabetic myocardial IRI, melatonin effectively improves mitochondrial quality control, alleviates diabetic cardiomyopathy, and hence reduces myocardial vulnerability to IRI through the SIRT1-PGC-1α or AMPK-PGC-1α pathway.166,699,700 The beneficial roles of melatonin in various toxin-caused organ injuries, such as chromium-induced lung injury, di-phthalate-induced granulosa cells apoptosis, CCl4-induced liver fibrosis, and rotenone-induced early porcine embryos, have been sufficiently demonstrated, which relies on the activation and increased expression of PGC-1α.701–704
Metformin
Metformin is currently the first-line and wide-spectrum drug treatment for T2DM and its inducible effect of AMPK is adequately documented. Thus, it is well established that metformin upregulates PGC-1α via AMPK phosphorylation under different experimental models.52 In the context of ischemic diseases occurring in the brain and heart, metformin pretreatment modulates mitochondrial energy metabolism and apoptotic cell death pathways through AMPK activation.705,706 When exposed to a high-glucose environment, metformin can promote INS-1 cell proliferation, enhance glucose-stimulated insulin secretion (GSIS), and suppress apoptosis by activating AMPK/SIRT1/PGC-1α signal pathway, up-regulating irisin expression, and inducing autophagy.707 Besides, metformin protects against gluco- and lipotoxicity-induced osteoblast apoptosis and reverses T2DM-associated deterioration in skeletal health, whereas depletion of PGC-1α abolishes this protective effect.708
Exercise training treatment
PGC-1α was acknowledged as a transcriptional coactivator induced by exercise as early as it was discovered.709–713 Terada et al. further illuminated that exercise stimulates PGC-1α expression at least via two distinct mechanisms, including AMPK activation and Ca2+ elevation.711 Moreover, the increased protein abundance in LKB1 and PGC-1α with endurance and interval training is responsible for maintaining the training-induced increases in mitochondrial mass.712 Exercise training has been confirmed to play important roles in muscle function, insulin sensitivity, mitochondrial biogenesis, angiogenesis, and unfolded protein response by regulating PGC-1α.214,714,715 Strikingly, PGC-1β declines rather than increases in prolonged exercise, which is more obvious when glycogen is not resynthesized to rest levels,716 in which the underlying mechanisms and causes are thought-provoking and need additional work to address. As Neto et al., published a wonderful review regarding the multifaceted and multi-systemic actions of physical exercise on PGC-1α signaling in just past 2023 April,717 we do not summarize the related frontier-of-knowledge data again herein.
Caloric restriction treatment
CR is a powerful and noninvasive intervention method to extend both life- and health span.718 PGC-1α, as a center of energy metabolism and mitochondrial OXPHOS, represents one of the most significant molecules that links the benefits of CR to the improvement of healthy conditions by limiting ROS generation, regulating insulin resistance, and mitochondrial function. The first and foremost investigation regarding the effects of CR in PGC-1α revealed that the levels of mtDNA, PGC-1α, NRF-1, and TFAM are upregulated in CR mice compared with ad libitum mice in adipose tissue, brain, heart, and liver.719 Soon afterward, Baker et al. reported that CR attenuates the decrement of PGC-1α gene expression with aging.720 Specifically, the potential mechanisms may involve that the suppression of GSK3β induced by CR to protect PGC-1α from intranuclear proteasomal degradation and the induction of SIRT1 by CR to enhance the transcriptional activity of PGC-1α.66 A subsequent series of research validated that CR upregulates the expression of PGC-1α as well as its target genes in mice, thereby supporting optimal energy metabolism and biochemical adaptation and performing protective roles in distinct diseases.721–725 However, another study found that CR downregulates the expression of the PPAR superfamily both in the muscle of normal and long-lived growth hormone receptor/binding protein knockout mice.726 In addition, the levels of PGC-1α, NRF-2, and ROS exhibit no alterations in rat liver of 40% restriction of dietary amino acids.727 More interestingly, short-term CR upregulates the mRNA levels of GLUT4, PGC-1α, and SIRT3 in cardiac muscles in young but not old rats, and downregulates only PGC-1α expression in skeletal muscles.728 Therefore, these conflictive results might be attributed to tissue type-dependent effects and age context-dependent influence of CR on PGC-1α. Moreover, the specific implementation plan, including varied caloric intake, variable feeding frequency, diet composition, and detection time point might also be partly responsible for the inconsistent phenomena.
Notably, although CR does not increase mitochondrial content, the adaptive induction of PGC-1α by CR maintains a functionally ‘efficient’ electron transport system and mitochondria in skeletal muscle, reflecting the importance of PGC-1α for the ability of dietary restriction to counteract the age-related decrement in mitochondrial respiration.723 Nevertheless, a normal improvement in glucose homeostasis in response to CR is observed in mice lacking skeletal muscle PGC-1α. Together with the results that muscle-specific overexpression of PGC-1α does not enhance metabolic improvements in response to CR, it is thought that skeletal muscle PGC-1α is not necessary for the whole-body benefits of CR.729,730 Obviously, consensus regarding the metabolic benefits of upregulated PGC-1α levels remains to be established. In other words, the reciprocity between PGC-1α levels, mitochondrial performance, and metabolic homeostasis may be more complex than previously, and more attention should be paid to decipher sophisticated interplay.
Conclusion remarks and future directions
Taken together, substantial insights into the PGC-1s family have illustrated their important functions and regulatory roles in the development of various diseases in the past few decades (Table 2, Fig. 8). Here, this review presents a complex regulatory network of the PGC-1s upstream, parallel, and downstream as well as the presently essential functions of PGC-1s, establishes an overview regarding the effects of PGC-1s in health and diseases, and introduces known therapeutic strategies targeting PGC-1s in pre-clinical experiments, which may thereby contribute to increasing our understanding of PGC-1s and tap the possible application of PGC-1s as novel therapeutic targets. Despite the encouraging progress in this area, some other directions in basic research and clinical applications of PGC-1s are worthy of attention.
Table 2.
A summary of the functions of PGC-1s in different organs and diseases models
| Disease models | Intervention | Main effects | Refs |
|---|---|---|---|
| Colorectal cancer | Intestinal-specific PGC-1β transfection | A peculiar intestinal morphology with very long villi and greater tumor susceptibility | 292 |
| Heart failure | Heart-specific PGC-1α knockout | Impairs mitochondrial respiration, energy metabolism, and Ca2+-handling and profound cardiac dysfunction | 361–363 |
| Heart failure | Heart-specific PGC-1α transfection | Increases mitochondrial damage and ROS insult | 366 |
| Peripartum cardiomyopathy | Heart-specific PGC-1α knockout | Enlarges left ventricular end-diastolic and end-systolic dimensions, and depresses cardiac contractile function | 390 |
| Parkinson’s disease | Dopaminergic neurons-specific transfection of PGC-1α | Elevates mitochondrial antioxidants and reduces loss of dopamine | 456 |
| Parkinson’s disease | Dopaminergic neurons-specific knockdown of PGC-1α | Leads to mitochondrial dysfunction | 459 |
| Parkinson’s disease | Microglial cells-specific knockdown of PGC-1α | Inhibits microglia activity, and reduces both M1 and M2 microglial activities. | 462 |
| Kidney fibrosis | Tubule-specific overexpression of PGC-1α | Alleviates mitochondrial morphology and FAO defect, and reduces apoptosis | 87 |
| Type 2 diabetes mellitus | Skeletal muscle-specific overexpression of PGC-1α | Upregulates expression of GLUT4 and increases glucose uptake in skeletal muscle- | 226,227 |
| Type 2 diabetes mellitus | β-cell-specific overexpression of PGC-1α | Decreases β-cell mass, and β-cell hypotrophy, decreases insulin secretion, and impairs glucose tolerance | 579 |
| Obesity | Adipose-specific PGC-1α knockout | Leads to insulin resistance and decreases the suppression of hepatic glucose output | 225 |
| Obesity | Adipose-specific overexpression of PGC-1α | Improves mitochondrial biogenesis and respiration, decreases fasting glucose, blood pressure, and fibrosis. | 590 |
Fig. 8.
The important regulatory roles of PGC-1α overexpression or activation in various organs. PGC-1α play important regulatory toles in various cellular events, including inflammation, apoptosis, mitochondrial function, and ROS generation, as well as some metabolic processes, including gluconeogenesis and glycogen stores in different organs or tissues, thus widely involving in the occurrence and progression of many diseases
Although the theme of this review is the PGC-1s family, PRC receives litter attention as the related research is few very much. Meanwhile, PGC-1β is also less relatively characterized compared to PGC-1α. Based on the current research, PGC-1α and PGC-1β have overlapping and distinct features and functions with each other. First, they manifest a similar expression pattern, as shown by extensively elevated expression in tissues demanding high energy requirements, such as the heart, skeletal muscle, and BAT. However, PGC-1α is highly inducible by different physiological or pharmacological cues, while PGC-1β seems to be less responsive to such stimuli. Second, PGC-1α can be regulated by several transcription and post-translational modifications, but the related report about PGC-1β is less. As sequence conservation among different members of the family, it can be inferred that many modulation modes of PGC-1α are also valid for PGC-1β and PRC. Last but not least, the functions between PGC-1α and PGC-1β are not always redundant. To be specific, both PGC-1α and PGC-1β significantly affect mitochondrial oxidative metabolism. Nevertheless, their functional heterogeneity is particularly evident in the liver. PGC-1α principally controls the gluconeogenesis genes, such as PEPCK and G6P, in response to fasting or feeding. Contrary to PGC-1α, PGC-1β predominately regulates hepatic lipid metabolism by interacting with ChREBP and SREBP.237,731 Therefore, a more complete understanding among different members of the PGC-1s family will be helpful for the development of innovative treatment.
As discussed above, except for the accepted double-edged sword of PGC-1s in cancer, upregulated PGC-1s expressions in other pathological processes are also not advantageous. For example, in the heart, sustaining physiological levels of PGC-1α expression following POH does not prevent mitochondrial and contractile dysfunction.376 However, even though the overexpression of PGC-1α is at a moderate level, enhanced mitochondrial biogenesis leads to significantly greater acute mortality in pressure-overloaded mouse hearts.366 Recently, Zhu et al. revealed that PGC-1α overexpression exacerbates cardiac degeneration and shortens lifespan in WT mice, but a favorable longevity-extending effect is observed in a third generation of telomerase-deficient mouse model.367 A similar conclusion also exists in the effects of PGC-1α on insulin resistance. Although it is widely recognized that PGC-1α is an important partner in combating insulin resistance,732 muscle-specific PGC-1α overexpression mice are more likely to develop insulin resistance, which comes from decreased insulin-stimulated muscle glucose uptake.733 Therefore, the following questions remain to be addressed in future investigations 1) probing the extent to which PGC-1α takes part in modulating energy homeostasis under physiological conditions, 2) exploring the mechanisms that PGC-1α activity alters in a diverse array of diseases, 3) determining the appropriate levels of PGC-1α to achieve health benefits under different pathologic condition, and 4) developing the methods to precisely tuning the expression of PGC-1α.
As for the clinical application of pharmacological methods targeting PGC-1α, some natural products like berberine, resveratrol, and curcumin, have shown protective effects in preclinical studies. However, they are still in a very embryonic state. Not only clinical trials but also multiple limitations of natural products such as low bioavailability, inadequate biological stability, and poor aqueous solubility, are needed to be further addressed. Additionally, these natural products have been widely reported to act on other targets, such as PI3K, AMPK, Nrf2, NF-κB, etc.734–736 Therefore, the observed therapeutic effects of these drugs might be unintended consequences rather than specific targeting of PGC-1s. The clinical drugs that have been approved, such as metformin and melatonin are promising candidates. The hurdle of expanding their clinical indications by targeting PGC-1α involves toxicology analyses, dosing, and formulation optimization. In addition, PGC-1α activator ZLN005 and inhibitor SR-18292 have been developed and applied in animal experiments,19,509,577,737–739 However, gaps and differences exist between rodent models and humans, thus more clinical trials are required.
Moreover, the pleiotropic effects of PGC-1α also depend on the tissue type. Specifically, the whole-body overexpression of human PGC-1α increases the expression of HNF4α and gluconeogenic enzymes PEPCK and G6P in the liver, and causes hepatic insulin resistance, while insulin sensitivity is improved in muscle.573 Likewise, short-term CR and endurance training differently affect energy metabolism and mitochondrial biogenesis in the cardiac and skeletal muscle.370,740 In one aspect, this emphasizes the necessity of conducting tissue-specific deficiency or overexpression models. In another aspect, from a therapeutic view, achieving targeted delivery to tissues or organs without affecting others contributes to avoiding unsatisfactory side effects. For example, Hao et al. designed 4,6-diamino-2-pyrimidinethiol-modified gold nanoparticles (D-Au NPs) and investigated its effect on intestinal mitochondria and studied the regulatory role of D-Au NPs on mitochondria metabolism-related disease. They found that D-Au NPs enhances the intestinal mechanical barrier by improving the antioxidation capability of mitochondria, and maintaining intestinal cellular homeostasis via the activation of AMPK and PGC-1α, as well as with its downstream signaling (UCP2 and DRP1).741 As described above, the cartilage-targeting dual-drug delivery nano platform (RB@MPMW) can achieve the sequential release of two agents (rapamycin and bilirubin) via near-infrared (NIR) laser irritation, thereby rescuing mitochondrial energy metabolism of chondrocytes via activating SIRT1-PGC-1α signaling pathway.546 More importantly, a nanoparticle that carries endothelial-specific PGC-1α expression plasmid was developed. Endothelial-specific overexpression of PGC-1α remarkably impedes endothelial to mesenchymal transition of pulmonary arterial endothelial cells and reduces vascular muscularization, thereby attenuating the development of pulmonary hypertension.742 With the development of drug screening technologies and targeted drug delivery systems, further investigations will facilitate improved applications of PGC-1α in clinical treatment.
With respect to the non-pharmacological methods mentioned above, making a personalized therapy plan based on a specific analysis and diagnosis of each individual is of vital importance. Of note, combined therapy is an emerging therapy and successfully alleviates the developments in animal models and clinical trials. For example, melatonin supplement integrated with exercise preserves mitochondrial function and represses oxidative stress, thus preventing cardiac injury.743 Besides, both CR in combination with high-intensity interval training and high-intensity interval training alone upregulates the levels of PPARγ and PGC-1α in visceral adipose tissue of obese rats, thus boosting the browning of visceral fat and ultimately weakening fat, while the former is more effective.744 Undeniably, a plausible strategy that combines moderate CR, physical activity, and pharmacological intervention represents one of the best ways to prevent diseases.
Interestingly, at 3 days post fertilization in zebrafish model, PGC-1α and PGC-1β knockdown decrease the transcript levels of citrate synthase, 3-hydroxyacyl-CoA dehydrogenase, and medium-chain acyl-coenzyme A dehydrogenase.745 Additionally, Kurchaba et al. discovered that the disruption of PGC-1α gene expression in striated muscle results in 4~fold increased mRNA levels of PGC-1α in mixed skeletal muscle and an opposite 4~fold downregulation in cardiac muscle. Meanwhile, two mitochondrial lipid transporters, CPT-1 and CPT-2, are strongly induced in mixed skeletal muscle and several transcriptional regulators (ERRα, NRF-1, and PGC-1β) are decreased without altering metabolic gene expression.746 This suggest that a mutation of PGC-1α promoter increases resting metabolism, translating into an enhanced mitochondrial oxidative capacity and FAO in adult zebrafish muscle.746 Therefore, zebrafish may serve as unique biomedical models for the investigation about the roles of PGC-1s in metabolic disorders.
In summary, the PGC-1s family is a promising target for the prevention and management of diseases. As big gaps of knowledge about the PGC-1s family still exist, especially about PGC-1β and PRC, more extensive research and the deeper elaborate mechanisms of other underlying roles for PGC-1s in the cellular events and pathological processes are hopefully warranted in the future.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (82360716, 82070422, and 82200330), China Postdoctoral Science Foundation (2023T160526 and 2022M722571), Research Plan Project of Shaanxi Institute of Basic Science (22JHQ053), High-end Foreign Expert Introduction Program of National Science and Technology (G2022040014L), Qinchuangyuan Traditional Chinese Medicine Innovation Research and Development Transformation Project (2022-QCYZH-036).
Author contributions
Y.Y., L.Q., and Y.L.Z. provided the conceptual idea and design of this study, wrote the manuscript and contributed equally to this work. C.D., Z.X.L., J.M.C., Y.C., and X.W. made the figures and tables. Y.Q.L, Y.T., and Y.Y provided valuable guidance and revised the paper. All authors have read and approved the article.
Competing interests
The authors declared no competing interests.
Footnotes
These authors contributed equally: Lu Qian, Yanli Zhu, Chao Deng.
References
- 1.Puigserver P, et al. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell. 1998;92:829–839. doi: 10.1016/S0092-8674(00)81410-5. [DOI] [PubMed] [Google Scholar]
- 2.Wu Z, et al. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell. 1999;98:115–124. doi: 10.1016/S0092-8674(00)80611-X. [DOI] [PubMed] [Google Scholar]
- 3.Lehman JJ, et al. Peroxisome proliferator-activated receptor gamma coactivator-1 promotes cardiac mitochondrial biogenesis. J. Clin. Invest. 2000;106:847–856. doi: 10.1172/JCI10268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu H, et al. Regulation of mitochondrial biogenesis in skeletal muscle by CaMK. Science. 2002;296:349–352. doi: 10.1126/science.1071163. [DOI] [PubMed] [Google Scholar]
- 5.Patten IS, et al. PGC-1 coactivators in the cardiovascular system. Trends Endocrinol. Metab. 2012;23:90–97. doi: 10.1016/j.tem.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 6.Rius-Perez, S. et al. Impairment of PGC-1 Alpha Up-Regulation Enhances Nitrosative Stress in the Liver during Acute Pancreatitis in Obese Mice. Antioxidants (Basel). 9, 887 (2020). [DOI] [PMC free article] [PubMed]
- 7.De Vitto H, et al. The PGC-1/ERR network and its role in precision oncology. NPJ Precis. Oncol. 2019;3:9. doi: 10.1038/s41698-019-0081-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yin Z, et al. MiR-30c/PGC-1beta protects against diabetic cardiomyopathy via PPARalpha. Cardiovasc. Diabetol. 2019;18:7. doi: 10.1186/s12933-019-0811-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Piccinin, E. et al. PGC-1s in the Spotlight with Parkinson’s Disease. Int J Mol Sci. 22, 3487 (2021). [DOI] [PMC free article] [PubMed]
- 10.Thirupathi A, et al. Multi-regulatory network of ROS: the interconnection of ROS, PGC-1 alpha, and AMPK-SIRT1. Exerc. J. Physiol. Biochem. 2017;73:487–494. doi: 10.1007/s13105-017-0576-y. [DOI] [PubMed] [Google Scholar]
- 11.Summermatter S, et al. PGC-1alpha and exercise in the control of body weight. Int. J. Obes. (Lond.). 2012;36:1428–1435. doi: 10.1038/ijo.2012.12. [DOI] [PubMed] [Google Scholar]
- 12.Suntar I, et al. Natural products, PGC-1 alpha, and Duchenne muscular dystrophy. Acta Pharm. Sin. B. 2020;10:734–745. doi: 10.1016/j.apsb.2020.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Puigserver P, et al. Activation of PPARgamma coactivator-1 through transcription factor docking. Science. 1999;286:1368–1371. doi: 10.1126/science.286.5443.1368. [DOI] [PubMed] [Google Scholar]
- 14.Herzig S, et al. CREB regulates hepatic gluconeogenesis through the coactivator PGC-1. Nature. 2001;413:179–183. doi: 10.1038/35093131. [DOI] [PubMed] [Google Scholar]
- 15.Vega RB, et al. The coactivator PGC-1 cooperates with peroxisome proliferator-activated receptor alpha in transcriptional control of nuclear genes encoding mitochondrial fatty acid oxidation enzymes. Mol. Cell Biol. 2000;20:1868–1876. doi: 10.1128/MCB.20.5.1868-1876.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin J, et al. Peroxisome proliferator-activated receptor gamma coactivator 1beta (PGC-1beta), a novel PGC-1-related transcription coactivator associated with host cell factor. J. Biol. Chem. 2002;277:1645–1648. doi: 10.1074/jbc.C100631200. [DOI] [PubMed] [Google Scholar]
- 17.Andersson U, et al. Pgc-1-related coactivator, a novel, serum-inducible coactivator of nuclear respiratory factor 1-dependent transcription in mammalian cells. Mol. Cell Biol. 2001;21:3738–3749. doi: 10.1128/MCB.21.11.3738-3749.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miura S, et al. Isoform-specific increases in murine skeletal muscle peroxisome proliferator-activated receptor-gamma coactivator-1alpha (PGC-1alpha) mRNA in response to beta2-adrenergic receptor activation and exercise. Endocrinology. 2008;149:4527–4533. doi: 10.1210/en.2008-0466. [DOI] [PubMed] [Google Scholar]
- 19.Zhang LN, et al. Novel small-molecule PGC-1alpha transcriptional regulator with beneficial effects on diabetic db/db mice. Diabetes. 2013;62:1297–1307. doi: 10.2337/db12-0703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kressler D, et al. The PGC-1-related protein PERC is a selective coactivator of estrogen receptor alpha. J. Biol. Chem. 2002;277:13918–13925. doi: 10.1074/jbc.M201134200. [DOI] [PubMed] [Google Scholar]
- 21.Handschin C, et al. Peroxisome proliferator-activated receptor gamma coactivator 1 coactivators, energy homeostasis, and metabolism. Endocr. Rev. 2006;27:728–735. doi: 10.1210/er.2006-0037. [DOI] [PubMed] [Google Scholar]
- 22.He X, et al. Peri-implantation lethality in mice lacking the PGC-1-related coactivator protein. Dev. Dyn. 2012;241:975–983. doi: 10.1002/dvdy.23769. [DOI] [PubMed] [Google Scholar]
- 23.Monsalve M, et al. Direct coupling of transcription and mRNA processing through the thermogenic coactivator PGC-1. Mol. Cell. 2000;6:307–316. doi: 10.1016/S1097-2765(00)00031-9. [DOI] [PubMed] [Google Scholar]
- 24.van Der Houven Van Oordt W, et al. Role of SR protein modular domains in alternative splicing specificity in vivo. Nucleic Acids Res. 2000;28:4822–4831. doi: 10.1093/nar/28.24.4822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Puigserver P, et al. Insulin-regulated hepatic gluconeogenesis through FOXO1-PGC-1alpha interaction. Nature. 2003;423:550–555. doi: 10.1038/nature01667. [DOI] [PubMed] [Google Scholar]
- 26.Cunningham JT, et al. mTOR controls mitochondrial oxidative function through a YY1-PGC-1alpha transcriptional complex. Nature. 2007;450:736–740. doi: 10.1038/nature06322. [DOI] [PubMed] [Google Scholar]
- 27.Scarpulla RC. Transcriptional paradigms in mammalian mitochondrial biogenesis and function. Physiol. Rev. 2008;88:611–638. doi: 10.1152/physrev.00025.2007. [DOI] [PubMed] [Google Scholar]
- 28.Haemmerle G, et al. ATGL-mediated fat catabolism regulates cardiac mitochondrial function via PPAR-alpha and PGC-1. Nat. Med. 2011;17:1076–1085. doi: 10.1038/nm.2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Muzio, G. et al. Peroxisome Proliferator-Activated Receptors (PPARs) and Oxidative Stress in Physiological Conditions and in Cancer. Antioxidants (Basel). 10, 1734 (2021). [DOI] [PMC free article] [PubMed]
- 30.Vernier M, et al. Estrogen-related receptors are targetable ROS sensors. Genes Dev. 2020;34:544–559. doi: 10.1101/gad.330746.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ziegler AK, et al. An anti-inflammatory phenotype in visceral adipose tissue of old lean mice, augmented by exercise. Sci. Rep. 2019;9:12069. doi: 10.1038/s41598-019-48587-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jager S, et al. AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1alpha. Proc. Natl Acad. Sci. Usa. 2007;104:12017–12022. doi: 10.1073/pnas.0705070104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gerhart-Hines Z, et al. Metabolic control of muscle mitochondrial function and fatty acid oxidation through SIRT1/PGC-1alpha. EMBO J. 2007;26:1913–1923. doi: 10.1038/sj.emboj.7601633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Teyssier C, et al. Activation of nuclear receptor coactivator PGC-1alpha by arginine methylation. Genes Dev. 2005;19:1466–1473. doi: 10.1101/gad.1295005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.De Rasmo D, et al. cAMP/Ca2+ response element-binding protein plays a central role in the biogenesis of respiratory chain proteins in mammalian cells. IUBMB Life. 2010;62:447–452. doi: 10.1002/iub.342. [DOI] [PubMed] [Google Scholar]
- 36.Ishii KA, et al. Coordination of PGC-1beta and iron uptake in mitochondrial biogenesis and osteoclast activation. Nat. Med. 2009;15:259–266. doi: 10.1038/nm.1910. [DOI] [PubMed] [Google Scholar]
- 37.Conkright MD, et al. TORCs: transducers of regulated CREB activity. Mol. Cell. 2003;12:413–423. doi: 10.1016/j.molcel.2003.08.013. [DOI] [PubMed] [Google Scholar]
- 38.Wu Z, et al. Transducer of regulated CREB-binding proteins (TORCs) induce PGC-1alpha transcription and mitochondrial biogenesis in muscle cells. Proc. Natl Acad. Sci. USA. 2006;103:14379–14384. doi: 10.1073/pnas.0606714103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nogueiras R, et al. Sirtuin 1 and sirtuin 3: physiological modulators of metabolism. Physiol. Rev. 2012;92:1479–1514. doi: 10.1152/physrev.00022.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rodgers JT, et al. Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1. Nature. 2005;434:113–118. doi: 10.1038/nature03354. [DOI] [PubMed] [Google Scholar]
- 41.Rodgers JT, et al. Fasting-dependent glucose and lipid metabolic response through hepatic sirtuin 1. Proc. Natl Acad. Sci. Usa. 2007;104:12861–12866. doi: 10.1073/pnas.0702509104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Aquilano K, et al. Extranuclear localization of SIRT1 and PGC-1alpha: an insight into possible roles in diseases associated with mitochondrial dysfunction. Curr. Mol. Med. 2013;13:140–154. doi: 10.2174/156652413804486241. [DOI] [PubMed] [Google Scholar]
- 43.Lerin C, et al. GCN5 acetyltransferase complex controls glucose metabolism through transcriptional repression of PGC-1alpha. Cell Metab. 2006;3:429–438. doi: 10.1016/j.cmet.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 44.Coste A, et al. The genetic ablation of SRC-3 protects against obesity and improves insulin sensitivity by reducing the acetylation of PGC-1alpha. Proc. Natl Acad. Sci. USA. 2008;105:17187–17192. doi: 10.1073/pnas.0808207105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kelly TJ, et al. GCN5-mediated transcriptional control of the metabolic coactivator PGC-1beta through lysine acetylation. J. Biol. Chem. 2009;284:19945–19952. doi: 10.1074/jbc.M109.015164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gonzalez A, et al. AMPK and TOR: The Yin and Yang of Cellular Nutrient Sensing and Growth Control. Cell Metab. 2020;31:472–492. doi: 10.1016/j.cmet.2020.01.015. [DOI] [PubMed] [Google Scholar]
- 47.Lin SC, et al. AMPK: Sensing Glucose as well as Cellular Energy Status. Cell Metab. 2018;27:299–313. doi: 10.1016/j.cmet.2017.10.009. [DOI] [PubMed] [Google Scholar]
- 48.Hardie DG, et al. AMPK: An Energy-Sensing Pathway with Multiple Inputs and Outputs. Trends Cell Biol. 2016;26:190–201. doi: 10.1016/j.tcb.2015.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Herzig S, et al. AMPK: guardian of metabolism and mitochondrial homeostasis. Nat. Rev. Mol. Cell Biol. 2018;19:121–135. doi: 10.1038/nrm.2017.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Suwa M, et al. Effects of chronic AICAR treatment on fiber composition, enzyme activity, UCP3, and PGC-1 in rat muscles. J. Appl Physiol. (1985). 2003;95:960–968. doi: 10.1152/japplphysiol.00349.2003. [DOI] [PubMed] [Google Scholar]
- 51.Irrcher I, et al. AMP-activated protein kinase-regulated activation of the PGC-1alpha promoter in skeletal muscle cells. PLoS One. 2008;3:e3614. doi: 10.1371/journal.pone.0003614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Suwa M, et al. Metformin increases the PGC-1alpha protein and oxidative enzyme activities possibly via AMPK phosphorylation in skeletal muscle in vivo. J. Appl Physiol. (1985). 2006;101:1685–1692. doi: 10.1152/japplphysiol.00255.2006. [DOI] [PubMed] [Google Scholar]
- 53.Canto C, et al. PGC-1alpha, SIRT1 and AMPK, an energy sensing network that controls energy expenditure. Curr. Opin. Lipidol. 2009;20:98–105. doi: 10.1097/MOL.0b013e328328d0a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Canto C, et al. Interdependence of AMPK and SIRT1 for metabolic adaptation to fasting and exercise in skeletal muscle. Cell Metab. 2010;11:213–219. doi: 10.1016/j.cmet.2010.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Canto C, et al. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature. 2009;458:1056–1060. doi: 10.1038/nature07813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Manning BD, et al. AKT/PKB Signaling: Navigating the Network. Cell. 2017;169:381–405. doi: 10.1016/j.cell.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rodgers JT, et al. Cdc2-like kinase 2 is an insulin-regulated suppressor of hepatic gluconeogenesis. Cell Metab. 2010;11:23–34. doi: 10.1016/j.cmet.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schultze SM, et al. Promiscuous affairs of PKB/AKT isoforms in metabolism. Arch. Physiol. Biochem. 2011;117:70–77. doi: 10.3109/13813455.2010.539236. [DOI] [PubMed] [Google Scholar]
- 59.Li X, et al. Akt/PKB regulates hepatic metabolism by directly inhibiting PGC-1alpha transcription coactivator. Nature. 2007;447:1012–1016. doi: 10.1038/nature05861. [DOI] [PubMed] [Google Scholar]
- 60.Santi SA, et al. Ablation of Akt2 induces autophagy through cell cycle arrest, the downregulation of p70S6K, and the deregulation of mitochondria in MDA-MB231 cells. PLoS One. 2011;6:e14614. doi: 10.1371/journal.pone.0014614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wright GL, et al. VEGF stimulation of mitochondrial biogenesis: requirement of AKT3 kinase. FASEB J. 2008;22:3264–3275. doi: 10.1096/fj.08-106468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Corum DG, et al. AKT3 controls mitochondrial biogenesis and autophagy via regulation of the major nuclear export protein CRM-1. FASEB J. 2014;28:395–407. doi: 10.1096/fj.13-235382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Larson-Casey JL, et al. Post-translational regulation of PGC-1alpha modulates fibrotic repair. FASEB J. 2021;35:e21675. doi: 10.1096/fj.202100339R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Beurel E, et al. Glycogen synthase kinase-3 (GSK3): regulation, actions, and diseases. Pharm. Ther. 2015;148:114–131. doi: 10.1016/j.pharmthera.2014.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Olson BL, et al. SCFCdc4 acts antagonistically to the PGC-1alpha transcriptional coactivator by targeting it for ubiquitin-mediated proteolysis. Genes Dev. 2008;22:252–264. doi: 10.1101/gad.1624208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Anderson RM, et al. Dynamic regulation of PGC-1alpha localization and turnover implicates mitochondrial adaptation in calorie restriction and the stress response. Aging Cell. 2008;7:101–111. doi: 10.1111/j.1474-9726.2007.00357.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Theeuwes WF, et al. Inactivation of glycogen synthase kinase 3beta (GSK-3beta) enhances mitochondrial biogenesis during myogenesis. Biochim Biophys. Acta Mol. Basis Dis. 2018;1864:2913–2926. doi: 10.1016/j.bbadis.2018.06.002. [DOI] [PubMed] [Google Scholar]
- 68.Theeuwes WF, et al. Inactivation of glycogen synthase kinase-3beta (GSK-3beta) enhances skeletal muscle oxidative metabolism. Biochim Biophys. Acta Mol. Basis Dis. 2017;1863:3075–3086. doi: 10.1016/j.bbadis.2017.09.018. [DOI] [PubMed] [Google Scholar]
- 69.Theeuwes WF, et al. Regulation of PGC-1alpha expression by a GSK-3beta-TFEB signaling axis in skeletal muscle. Biochim Biophys. Acta Mol. Cell Res. 2020;1867:118610. doi: 10.1016/j.bbamcr.2019.118610. [DOI] [PubMed] [Google Scholar]
- 70.Goo HG, et al. Pathogenic Role of Serine Protease HtrA2/Omi in Neurodegenerative Diseases. Curr. Protein Pept. Sci. 2017;18:746–757. doi: 10.2174/1389203717666160311115750. [DOI] [PubMed] [Google Scholar]
- 71.Xu R, et al. The protease Omi regulates mitochondrial biogenesis through the GSK3beta/PGC-1alpha pathway. Cell Death Dis. 2014;5:e1373. doi: 10.1038/cddis.2014.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wu, L. et al. Hepatic Gadd45beta promotes hyperglycemia and glucose intolerance through DNA demethylation of PGC-1alpha. J Exp Med. 218, e20201475 (2021). [DOI] [PMC free article] [PubMed]
- 73.Ribel-Madsen R, et al. Genome-wide analysis of DNA methylation differences in muscle and fat from monozygotic twins discordant for type 2 diabetes. PLoS One. 2012;7:e51302. doi: 10.1371/journal.pone.0051302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ling C, et al. Epigenetic regulation of PPARGC1A in human type 2 diabetic islets and effect on insulin secretion. Diabetologia. 2008;51:615–622. doi: 10.1007/s00125-007-0916-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sookoian S, et al. Epigenetic regulation of insulin resistance in nonalcoholic fatty liver disease: impact of liver methylation of the peroxisome proliferator-activated receptor gamma coactivator 1alpha promoter. Hepatology. 2010;52:1992–2000. doi: 10.1002/hep.23927. [DOI] [PubMed] [Google Scholar]
- 76.Bajpeyi S, et al. Skeletal Muscle PGC1alpha -1 Nucleosome Position and -260 nt DNA Methylation Determine Exercise Response and Prevent Ectopic Lipid Accumulation in Men. Endocrinology. 2017;158:2190–2199. doi: 10.1210/en.2017-00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yan H, et al. Adeno-associated virus-mediated delivery of anti-miR-199a tough decoys attenuates cardiac hypertrophy by targeting PGC-1alpha. Mol. Ther. Nucleic Acids. 2021;23:406–417. doi: 10.1016/j.omtn.2020.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Du J, et al. Inhibition of miR-23a attenuates doxorubicin-induced mitochondria-dependent cardiomyocyte apoptosis by targeting the PGC-1alpha/Drp1 pathway. Toxicol. Appl. Pharmacol. 2019;369:73–81. doi: 10.1016/j.taap.2019.02.016. [DOI] [PubMed] [Google Scholar]
- 79.Zhang S, et al. PGC-1 alpha interacts with microRNA-217 to functionally regulate breast cancer cell proliferation. Biomed. Pharmacother. 2017;85:541–548. doi: 10.1016/j.biopha.2016.11.062. [DOI] [PubMed] [Google Scholar]
- 80.Xue Y, et al. MicroRNA-19b/221/222 induces endothelial cell dysfunction via suppression of PGC-1alpha in the progression of atherosclerosis. Atherosclerosis. 2015;241:671–681. doi: 10.1016/j.atherosclerosis.2015.06.031. [DOI] [PubMed] [Google Scholar]
- 81.Sun LY, et al. MicroRNA-23a mediates mitochondrial compromise in estrogen deficiency-induced concentric remodeling via targeting PGC-1alpha. J. Mol. Cell Cardiol. 2014;75:1–11. doi: 10.1016/j.yjmcc.2014.06.012. [DOI] [PubMed] [Google Scholar]
- 82.Liang J, et al. MicroRNA-29a-c decrease fasting blood glucose levels by negatively regulating hepatic gluconeogenesis. J. Hepatol. 2013;58:535–542. doi: 10.1016/j.jhep.2012.10.024. [DOI] [PubMed] [Google Scholar]
- 83.Wang B, et al. Stat3-mediated activation of microRNA-23a suppresses gluconeogenesis in hepatocellular carcinoma by down-regulating glucose-6-phosphatase and peroxisome proliferator-activated receptor gamma, coactivator 1 alpha. Hepatology. 2012;56:186–197. doi: 10.1002/hep.25632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fang Z, et al. miR-696 plays a role in hepatic gluconeogenesis in ob/ob mice by targeting PGC-1alpha. Int J. Mol. Med. 2016;38:845–852. doi: 10.3892/ijmm.2016.2659. [DOI] [PubMed] [Google Scholar]
- 85.Aoi W, et al. The microRNA miR-696 regulates PGC-1alpha in mouse skeletal muscle in response to physical activity. Am. J. Physiol. Endocrinol. Metab. 2010;298:E799–E806. doi: 10.1152/ajpendo.00448.2009. [DOI] [PubMed] [Google Scholar]
- 86.Yadav H, et al. Protection from obesity and diabetes by blockade of TGF-beta/Smad3 signaling. Cell Metab. 2011;14:67–79. doi: 10.1016/j.cmet.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Han SH, et al. PGC-1alpha Protects from Notch-Induced Kidney Fibrosis Development. J. Am. Soc. Nephrol. 2017;28:3312–3322. doi: 10.1681/ASN.2017020130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lustig Y, et al. Separation of the gluconeogenic and mitochondrial functions of PGC-1alpha through S6 kinase. Genes Dev. 2011;25:1232–1244. doi: 10.1101/gad.2054711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ruan HB, et al. O-GlcNAc transferase/host cell factor C1 complex regulates gluconeogenesis by modulating PGC-1alpha stability. Cell Metab. 2012;16:226–237. doi: 10.1016/j.cmet.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rytinki MM, et al. SUMOylation attenuates the function of PGC-1alpha. J. Biol. Chem. 2009;284:26184–26193. doi: 10.1074/jbc.M109.038943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cai R, et al. SUMO-specific protease 1 regulates mitochondrial biogenesis through PGC-1alpha. J. Biol. Chem. 2012;287:44464–44470. doi: 10.1074/jbc.M112.422626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Trausch-Azar J, et al. Ubiquitin proteasome-dependent degradation of the transcriptional coactivator PGC-1alpha via the N-terminal. Pathw. J. Biol. Chem. 2010;285:40192–40200. doi: 10.1074/jbc.M110.131615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Fujita H, et al. The E3 ligase synoviolin controls body weight and mitochondrial biogenesis through negative regulation of PGC-1beta. EMBO J. 2015;34:1042–1055. doi: 10.15252/embj.201489897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Oberkofler H, et al. Potentiation of liver X receptor transcriptional activity by peroxisome-proliferator-activated receptor gamma co-activator 1 alpha. Biochem. J. 2003;371:89–96. doi: 10.1042/bj20021665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhang Y, et al. Peroxisome proliferator-activated receptor-gamma coactivator 1alpha (PGC-1alpha) regulates triglyceride metabolism by activation of the nuclear receptor FXR. Genes Dev. 2004;18:157–169. doi: 10.1101/gad.1138104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Safi R, et al. Coactivation of liver receptor homologue-1 by peroxisome proliferator-activated receptor gamma coactivator-1alpha on aromatase promoter II and its inhibition by activated retinoid X receptor suggest a novel target for breast-specific antiestrogen therapy. Cancer Res. 2005;65:11762–11770. doi: 10.1158/0008-5472.CAN-05-2792. [DOI] [PubMed] [Google Scholar]
- 97.Beaudry JB, et al. Threshold levels of hepatocyte nuclear factor 6 (HNF-6) acting in synergy with HNF-4 and PGC-1alpha are required for time-specific gene expression during liver development. Mol. Cell Biol. 2006;26:6037–6046. doi: 10.1128/MCB.02445-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Savkur RS, et al. Ligand-dependent coactivation of the human bile acid receptor FXR by the peroxisome proliferator-activated receptor gamma coactivator-1alpha. J. Pharm. Exp. Ther. 2005;312:170–178. doi: 10.1124/jpet.104.072124. [DOI] [PubMed] [Google Scholar]
- 99.Jang WG, et al. Glucocorticoid receptor mediated repression of human insulin gene expression is regulated by PGC-1alpha. Biochem Biophys. Res. Commun. 2007;352:716–721. doi: 10.1016/j.bbrc.2006.11.074. [DOI] [PubMed] [Google Scholar]
- 100.Issemann I, et al. Activation of a member of the steroid hormone receptor superfamily by peroxisome proliferators. Nature. 1990;347:645–650. doi: 10.1038/347645a0. [DOI] [PubMed] [Google Scholar]
- 101.Finck BN. The PPAR regulatory system in cardiac physiology and disease. Cardiovasc. Res. 2007;73:269–277. doi: 10.1016/j.cardiores.2006.08.023. [DOI] [PubMed] [Google Scholar]
- 102.Finck BN, et al. Lipin 1 is an inducible amplifier of the hepatic PGC-1alpha/PPARalpha regulatory pathway. Cell Metab. 2006;4:199–210. doi: 10.1016/j.cmet.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 103.Lehman JJ, et al. Transcriptional activation of energy metabolic switches in the developing and hypertrophied heart. Clin. Exp. Pharm. Physiol. 2002;29:339–345. doi: 10.1046/j.1440-1681.2002.03655.x. [DOI] [PubMed] [Google Scholar]
- 104.Li Y, et al. Structural and biochemical basis for the binding selectivity of peroxisome proliferator-activated receptor gamma to PGC-1alpha. J. Biol. Chem. 2008;283:19132–19139. doi: 10.1074/jbc.M802040200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hondares E, et al. Peroxisome proliferator-activated receptor alpha (PPARalpha) induces PPARgamma coactivator 1alpha (PGC-1alpha) gene expression and contributes to thermogenic activation of brown fat: involvement of PRDM1. 6. J. Biol. Chem. 2011;286:43112–43122. doi: 10.1074/jbc.M111.252775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Pan D, et al. Twist-1 is a PPARdelta-inducible, negative-feedback regulator of PGC-1alpha in brown fat metabolism. Cell. 2009;137:73–86. doi: 10.1016/j.cell.2009.01.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kleiner S, et al. PPARdelta agonism activates fatty acid oxidation via PGC-1alpha but does not increase mitochondrial gene expression and function. J. Biol. Chem. 2009;284:18624–18633. doi: 10.1074/jbc.M109.008797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Koh JH, et al. PPARbeta Is Essential for Maintaining Normal Levels of PGC-1alpha and Mitochondria and for the Increase in Muscle Mitochondria Induced by Exercise. Cell Metab. 2017;25:1176–1185.e1175. doi: 10.1016/j.cmet.2017.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zhang Y, et al. PGC-1alpha induces apoptosis in human epithelial ovarian cancer cells through a PPARgamma-dependent pathway. Cell Res. 2007;17:363–373. doi: 10.1038/cr.2007.11. [DOI] [PubMed] [Google Scholar]
- 110.Katsouri L, et al. PPARgamma co-activator-1alpha (PGC-1alpha) reduces amyloid-beta generation through a PPARgamma-dependent. mechanism. J. Alzheimers Dis. 2011;25:151–162. doi: 10.3233/JAD-2011-101356. [DOI] [PubMed] [Google Scholar]
- 111.Barroso E, et al. The PPARbeta/delta activator GW501516 prevents the down-regulation of AMPK caused by a high-fat diet in liver and amplifies the PGC-1alpha-Lipin 1-PPARalpha pathway leading to increased fatty acid oxidation. Endocrinology. 2011;152:1848–1859. doi: 10.1210/en.2010-1468. [DOI] [PubMed] [Google Scholar]
- 112.Giguere V. To ERR in the estrogen pathway. Trends Endocrinol. Metab. 2002;13:220–225. doi: 10.1016/S1043-2760(02)00592-1. [DOI] [PubMed] [Google Scholar]
- 113.Huss JM, et al. Peroxisome proliferator-activated receptor coactivator-1alpha (PGC-1alpha) coactivates the cardiac-enriched nuclear receptors estrogen-related receptor-alpha and -gamma. Identification of novel leucine-rich interaction motif within PGC-1alpha. J. Biol. Chem. 2002;277:40265–40274. doi: 10.1074/jbc.M206324200. [DOI] [PubMed] [Google Scholar]
- 114.Schreiber SN, et al. The transcriptional coactivator PGC-1 regulates the expression and activity of the orphan nuclear receptor estrogen-related receptor alpha (ERRalpha) J. Biol. Chem. 2003;278:9013–9018. doi: 10.1074/jbc.M212923200. [DOI] [PubMed] [Google Scholar]
- 115.Schreiber SN, et al. The estrogen-related receptor alpha (ERRalpha) functions in PPARgamma coactivator 1alpha (PGC-1alpha)-induced mitochondrial biogenesis. Proc. Natl Acad. Sci. USA. 2004;101:6472–6477. doi: 10.1073/pnas.0308686101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Willy PJ, et al. Regulation of PPARgamma coactivator 1alpha (PGC-1alpha) signaling by an estrogen-related receptor alpha (ERRalpha) ligand. Proc. Natl Acad. Sci. USA. 2004;101:8912–8917. doi: 10.1073/pnas.0401420101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Huss JM, et al. Estrogen-related receptor alpha directs peroxisome proliferator-activated receptor alpha signaling in the transcriptional control of energy metabolism in cardiac and skeletal muscle. Mol. Cell Biol. 2004;24:9079–9091. doi: 10.1128/MCB.24.20.9079-9091.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wende AR, et al. PGC-1alpha coactivates PDK4 gene expression via the orphan nuclear receptor ERRalpha: a mechanism for transcriptional control of muscle glucose metabolism. Mol. Cell Biol. 2005;25:10684–10694. doi: 10.1128/MCB.25.24.10684-10694.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Huss JM, et al. The nuclear receptor ERRalpha is required for the bioenergetic and functional adaptation to cardiac pressure overload. Cell Metab. 2007;6:25–37. doi: 10.1016/j.cmet.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 120.Wang H, et al. Estrogen-related receptor alpha interacts cooperatively with peroxisome proliferator-activated receptor-gamma coactivator-1alpha to regulate osteocalcin gene expression. Cell Biol. Int. 2013;37:1259–1265. doi: 10.1002/cbin.10148. [DOI] [PubMed] [Google Scholar]
- 121.Lai L, et al. A role for peroxisome proliferator-activated receptor gamma coactivator 1 (PGC-1) in the regulation of cardiac mitochondrial phospholipid biosynthesis. J. Biol. Chem. 2014;289:2250–2259. doi: 10.1074/jbc.M113.523654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Brown EL, et al. PGC-1alpha and PGC-1beta increase CrT expression and creatine uptake in myotubes via ERRalpha. Biochim Biophys. Acta. 2014;1843:2937–2943. doi: 10.1016/j.bbamcr.2014.08.010. [DOI] [PubMed] [Google Scholar]
- 123.Barroso WA, et al. High-fat diet inhibits PGC-1alpha suppressive effect on NFkappaB signaling in hepatocytes. Eur. J. Nutr. 2018;57:1891–1900. doi: 10.1007/s00394-017-1472-5. [DOI] [PubMed] [Google Scholar]
- 124.McMeekin LJ, et al. Estrogen-related Receptor Alpha (ERRalpha) is Required for PGC-1alpha-dependent Gene Expression in the Mouse Brain. Neuroscience. 2021;479:70–90. doi: 10.1016/j.neuroscience.2021.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Arany Z, et al. HIF-independent regulation of VEGF and angiogenesis by the transcriptional coactivator PGC-1alpha. Nature. 2008;451:1008–1012. doi: 10.1038/nature06613. [DOI] [PubMed] [Google Scholar]
- 126.Brown EL, et al. PGC-1alpha and PGC-1beta Increase Protein Synthesis via ERRalpha in C2C12 Myotubes. Front Physiol. 2018;9:1336. doi: 10.3389/fphys.2018.01336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Gacias M, et al. PGC-1beta regulates mouse carnitine-acylcarnitine translocase through estrogen-related receptor alpha. Biochem Biophys. Res Commun. 2012;423:838–843. doi: 10.1016/j.bbrc.2012.06.051. [DOI] [PubMed] [Google Scholar]
- 128.Kamei Y, et al. PPARgamma coactivator 1beta/ERR ligand 1 is an ERR protein ligand, whose expression induces a high-energy expenditure and antagonizes obesity. Proc. Natl Acad. Sci. USA. 2003;100:12378–12383. doi: 10.1073/pnas.2135217100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wang L, et al. PGC-1alpha induces dynamic protein interactions on the ERRalpha gene multi-hormone response element nucleosome in kidney cells. Biochem. J. 2008;416:407–419. doi: 10.1042/BJ20081085. [DOI] [PubMed] [Google Scholar]
- 130.Virbasius CA, et al. NRF-1, an activator involved in nuclear-mitochondrial interactions, utilizes a new DNA-binding domain conserved in a family of developmental regulators. Genes Dev. 1993;7:2431–2445. doi: 10.1101/gad.7.12a.2431. [DOI] [PubMed] [Google Scholar]
- 131.Virbasius JV, et al. Identity of GABP with NRF-2, a multisubunit activator of cytochrome oxidase expression, reveals a cellular role for an ETS domain activator of viral promoters. Genes Dev. 1993;7:380–392. doi: 10.1101/gad.7.3.380. [DOI] [PubMed] [Google Scholar]
- 132.Huo L, et al. Mitochondrial DNA instability and peri-implantation lethality associated with targeted disruption of nuclear respiratory factor 1 in mice. Mol. Cell Biol. 2001;21:644–654. doi: 10.1128/MCB.21.2.644-654.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Scarpulla RC. Transcriptional activators and coactivators in the nuclear control of mitochondrial function in mammalian cells. Gene. 2002;286:81–89. doi: 10.1016/S0378-1119(01)00809-5. [DOI] [PubMed] [Google Scholar]
- 134.Gleyzer N, et al. Control of mitochondrial transcription specificity factors (TFB1M and TFB2M) by nuclear respiratory factors (NRF-1 and NRF-2) and PGC-1 family coactivators. Mol. Cell Biol. 2005;25:1354–1366. doi: 10.1128/MCB.25.4.1354-1366.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Vercauteren K, et al. PGC-1-related coactivator complexes with HCF-1 and NRF-2beta in mediating NRF-2(GABP)-dependent respiratory gene expression. J. Biol. Chem. 2008;283:12102–12111. doi: 10.1074/jbc.M710150200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Scarpulla RC. Nuclear control of respiratory chain expression by nuclear respiratory factors and PGC-1-related coactivator. Ann. N. Y. Acad. Sci. 2008;1147:321–334. doi: 10.1196/annals.1427.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Baar K. Involvement of PPAR gamma co-activator-1, nuclear respiratory factors 1 and 2, and PPAR alpha in the adaptive response to endurance exercise. Proc. Nutr. Soc. 2004;63:269–273. doi: 10.1079/PNS2004334. [DOI] [PubMed] [Google Scholar]
- 138.Lau HH, et al. The molecular functions of hepatocyte nuclear factors - In and beyond the liver. J. Hepatol. 2018;68:1033–1048. doi: 10.1016/j.jhep.2017.11.026. [DOI] [PubMed] [Google Scholar]
- 139.Rhee J, et al. Regulation of hepatic fasting response by PPARgamma coactivator-1alpha (PGC-1): requirement for hepatocyte nuclear factor 4alpha in gluconeogenesis. Proc. Natl Acad. Sci. USA. 2003;100:4012–4017. doi: 10.1073/pnas.0730870100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Bhalla S, et al. Ligand-activated pregnane X receptor interferes with HNF-4 signaling by targeting a common coactivator PGC-1alpha. Functional implications in hepatic cholesterol and glucose metabolism. J. Biol. Chem. 2004;279:45139–45147. doi: 10.1074/jbc.M405423200. [DOI] [PubMed] [Google Scholar]
- 141.Ponugoti B, et al. Functional interaction of hepatic nuclear factor-4 and peroxisome proliferator-activated receptor-gamma coactivator 1alpha in CYP7A1 regulation is inhibited by a key lipogenic activator, sterol regulatory element-binding protein-1c. Mol. Endocrinol. 2007;21:2698–2712. doi: 10.1210/me.2007-0196. [DOI] [PubMed] [Google Scholar]
- 142.Dietrich CG, et al. Fasting induces basolateral uptake transporters of the SLC family in the liver via HNF4alpha and PGC1alpha. Am. J. Physiol. Gastrointest. Liver Physiol. 2007;293:G585–G590. doi: 10.1152/ajpgi.00175.2007. [DOI] [PubMed] [Google Scholar]
- 143.Ding X, et al. Regulation of constitutive androstane receptor and its target genes by fasting, cAMP, hepatocyte nuclear factor alpha, and the coactivator peroxisome proliferator-activated receptor gamma coactivator-1alpha. J. Biol. Chem. 2006;281:26540–26551. doi: 10.1074/jbc.M600931200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Rhee J, et al. Partnership of PGC-1alpha and HNF4alpha in the regulation of lipoprotein metabolism. J. Biol. Chem. 2006;281:14683–14690. doi: 10.1074/jbc.M512636200. [DOI] [PubMed] [Google Scholar]
- 145.Wang Z, et al. Modulation of hepatocyte nuclear factor-4alpha function by the peroxisome-proliferator-activated receptor-gamma co-activator-1alpha in the acute-phase response. Biochem. J. 2008;415:289–296. doi: 10.1042/BJ20080355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kummer E, et al. Mechanisms and regulation of protein synthesis in mitochondria. Nat. Rev. Mol. Cell Biol. 2021;22:307–325. doi: 10.1038/s41580-021-00332-2. [DOI] [PubMed] [Google Scholar]
- 147.Leduc-Gaudet, J. P. et al. Mitochondrial Dynamics and Mitophagy in Skeletal Muscle Health and Aging. Int. J. Mol. Sci. 22, 8179 (2021). [DOI] [PMC free article] [PubMed]
- 148.Barrera MJ, et al. Dysfunctional mitochondria as critical players in the inflammation of autoimmune diseases: Potential role in Sjogren’s syndrome. Autoimmun. Rev. 2021;20:102867. doi: 10.1016/j.autrev.2021.102867. [DOI] [PubMed] [Google Scholar]
- 149.Kumar AA, et al. Mitochondrial Dysfunction in Heart Failure With Preserved Ejection Fraction. Circulation. 2019;139:1435–1450. doi: 10.1161/CIRCULATIONAHA.118.036259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Popov LD. Mitochondrial biogenesis: An update. J. Cell Mol. Med. 2020;24:4892–4899. doi: 10.1111/jcmm.15194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Diaz F, et al. Mitochondrial biogenesis and turnover. Cell Calcium. 2008;44:24–35. doi: 10.1016/j.ceca.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kelly DP, et al. Transcriptional regulatory circuits controlling mitochondrial biogenesis and function. Genes Dev. 2004;18:357–368. doi: 10.1101/gad.1177604. [DOI] [PubMed] [Google Scholar]
- 153.Choi YS, et al. Impaired coactivator activity of the Gly482 variant of peroxisome proliferator-activated receptor gamma coactivator-1alpha (PGC-1alpha) on mitochondrial transcription factor A (Tfam) promoter. Biochem Biophys. Res Commun. 2006;344:708–712. doi: 10.1016/j.bbrc.2006.03.193. [DOI] [PubMed] [Google Scholar]
- 154.Srivastava S, et al. PGC-1alpha/beta induced expression partially compensates for respiratory chain defects in cells from patients with mitochondrial disorders. Hum. Mol. Genet. 2009;18:1805–1812. doi: 10.1093/hmg/ddp093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Pernas L, et al. Mito-Morphosis: Mitochondrial Fusion, Fission, and Cristae Remodeling as Key Mediators of Cellular Function. Annu Rev. Physiol. 2016;78:505–531. doi: 10.1146/annurev-physiol-021115-105011. [DOI] [PubMed] [Google Scholar]
- 156.Al Ojaimi, M. et al. Mitochondrial Fission and Fusion: Molecular Mechanisms, Biological Functions, and Related Disorders. Membranes (Basel). 12, 893 (2022). [DOI] [PMC free article] [PubMed]
- 157.Sabouny R, et al. Reciprocal Regulation of Mitochondrial Fission and Fusion. Trends Biochem Sci. 2020;45:564–577. doi: 10.1016/j.tibs.2020.03.009. [DOI] [PubMed] [Google Scholar]
- 158.Quiles JM, et al. The role of mitochondrial fission in cardiovascular health and disease. Nat. Rev. Cardiol. 2022;19:723–736. doi: 10.1038/s41569-022-00703-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Soriano FX, et al. Evidence for a mitochondrial regulatory pathway defined by peroxisome proliferator-activated receptor-gamma coactivator-1 alpha, estrogen-related receptor-alpha, and mitofusin 2. Diabetes. 2006;55:1783–1791. doi: 10.2337/db05-0509. [DOI] [PubMed] [Google Scholar]
- 160.Martin OJ, et al. A role for peroxisome proliferator-activated receptor gamma coactivator-1 in the control of mitochondrial dynamics during postnatal cardiac growth. Circ. Res. 2014;114:626–636. doi: 10.1161/CIRCRESAHA.114.302562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Cannavino J, et al. The role of alterations in mitochondrial dynamics and PGC-1alpha over-expression in fast muscle atrophy following hindlimb unloading. J. Physiol. 2015;593:1981–1995. doi: 10.1113/jphysiol.2014.286740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Cowell RM, et al. Regulation of PGC-1alpha and PGC-1alpha-responsive genes with forskolin-induced Schwann cell differentiation. Neurosci. Lett. 2008;439:269–274. doi: 10.1016/j.neulet.2008.04.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Zechner C, et al. Total skeletal muscle PGC-1 deficiency uncouples mitochondrial derangements from fiber type determination and insulin sensitivity. Cell Metab. 2010;12:633–642. doi: 10.1016/j.cmet.2010.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Halling JF, et al. Exercise training protects against aging-induced mitochondrial fragmentation in mouse skeletal muscle in a PGC-1alpha dependent manner. Exp. Gerontol. 2017;96:1–6. doi: 10.1016/j.exger.2017.05.020. [DOI] [PubMed] [Google Scholar]
- 165.Halling JF, et al. PGC-1alpha regulates mitochondrial properties beyond biogenesis with aging and exercise training. Am. J. Physiol. Endocrinol. Metab. 2019;317:E513–E525. doi: 10.1152/ajpendo.00059.2019. [DOI] [PubMed] [Google Scholar]
- 166.Ding M, et al. Melatonin prevents Drp1-mediated mitochondrial fission in diabetic hearts through SIRT1-PGC1alpha pathway. J. Pineal Res. 2018;65:e12491. doi: 10.1111/jpi.12491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Peng K, et al. The Interaction of Mitochondrial Biogenesis and Fission/Fusion Mediated by PGC-1alpha Regulates Rotenone-Induced Dopaminergic Neurotoxicity. Mol. Neurobiol. 2017;54:3783–3797. doi: 10.1007/s12035-016-9944-9. [DOI] [PubMed] [Google Scholar]
- 168.Sui YB, et al. Shen Qi Li Xin formula improves chronic heart failure through balancing mitochondrial fission and fusion via upregulation of PGC-1alpha. J. Physiol. Sci. 2021;71:32. doi: 10.1186/s12576-021-00816-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Bravo-San Pedro JM, et al. Autophagy and Mitophagy in Cardiovascular Disease. Circ. Res. 2017;120:1812–1824. doi: 10.1161/CIRCRESAHA.117.311082. [DOI] [PubMed] [Google Scholar]
- 170.Vainshtein A, et al. PGC-1alpha modulates denervation-induced mitophagy in skeletal muscle. Skelet. Muscle. 2015;5:9. doi: 10.1186/s13395-015-0033-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Liu W, et al. Positive regulation of TFEB and mitophagy by PGC-1alpha to alleviate LPS-induced acute lung injury in rats. Biochem Biophys. Res Commun. 2021;577:1–5. doi: 10.1016/j.bbrc.2021.08.064. [DOI] [PubMed] [Google Scholar]
- 172.Vainshtein A, et al. Role of PGC-1alpha during acute exercise-induced autophagy and mitophagy in skeletal muscle. Am. J. Physiol. Cell Physiol. 2015;308:C710–C719. doi: 10.1152/ajpcell.00380.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Liu L, et al. Mitophagy receptor FUNDC1 is regulated by PGC-1alpha/NRF1 to fine tune mitochondrial homeostasis. EMBO Rep. 2021;22:e50629. doi: 10.15252/embr.202050629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Nguyen TN, et al. Deciphering the Molecular Signals of PINK1/Parkin Mitophagy. Trends Cell Biol. 2016;26:733–744. doi: 10.1016/j.tcb.2016.05.008. [DOI] [PubMed] [Google Scholar]
- 175.Peng K, et al. Mutual Antagonism of PINK1/Parkin and PGC-1alpha Contributes to Maintenance of Mitochondrial Homeostasis in Rotenone-Induced Neurotoxicity. Neurotox. Res. 2019;35:331–343. doi: 10.1007/s12640-018-9957-4. [DOI] [PubMed] [Google Scholar]
- 176.Gao CL, et al. Overexpression of PGC-1beta improves insulin sensitivity and mitochondrial function in 3T3-L1 adipocytes. Mol. Cell Biochem. 2011;353:215–223. doi: 10.1007/s11010-011-0789-2. [DOI] [PubMed] [Google Scholar]
- 177.Lelliott CJ, et al. Ablation of PGC-1beta results in defective mitochondrial activity, thermogenesis, hepatic function, and cardiac performance. PLoS Biol. 2006;4:e369. doi: 10.1371/journal.pbio.0040369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Li S, et al. Reduced PGC-1beta protein expression may underlie corticosterone inhibition of mitochondrial biogenesis and oxidative phosphorylation in chicken muscles. Front Physiol. 2022;13:989547. doi: 10.3389/fphys.2022.989547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Vianna CR, et al. Hypomorphic mutation of PGC-1beta causes mitochondrial dysfunction and liver insulin resistance. Cell Metab. 2006;4:453–464. doi: 10.1016/j.cmet.2006.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Gali Ramamoorthy T, et al. The transcriptional coregulator PGC-1beta controls mitochondrial function and anti-oxidant defence in skeletal muscles. Nat. Commun. 2015;6:10210. doi: 10.1038/ncomms10210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Sies H, et al. Oxidative Stress. Annu Rev. Biochem. 2017;86:715–748. doi: 10.1146/annurev-biochem-061516-045037. [DOI] [PubMed] [Google Scholar]
- 182.St-Pierre J, et al. Suppression of reactive oxygen species and neurodegeneration by the PGC-1 transcriptional coactivators. Cell. 2006;127:397–408. doi: 10.1016/j.cell.2006.09.024. [DOI] [PubMed] [Google Scholar]
- 183.Valle I, et al. PGC-1alpha regulates the mitochondrial antioxidant defense system in vascular endothelial cells. Cardiovasc. Res. 2005;66:562–573. doi: 10.1016/j.cardiores.2005.01.026. [DOI] [PubMed] [Google Scholar]
- 184.Iacovelli J, et al. PGC-1alpha Induces Human RPE Oxidative Metabolism and Antioxidant Capacity. Invest. Ophthalmol. Vis. Sci. 2016;57:1038–1051. doi: 10.1167/iovs.15-17758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Sanchez-Ramos C, et al. PGC-1alpha regulates translocated in liposarcoma activity: role in oxidative stress gene expression. Antioxid. Redox Signal. 2011;15:325–337. doi: 10.1089/ars.2010.3643. [DOI] [PubMed] [Google Scholar]
- 186.Olmos Y, et al. Mutual dependence of Foxo3a and PGC-1alpha in the induction of oxidative stress genes. J. Biol. Chem. 2009;284:14476–14484. doi: 10.1074/jbc.M807397200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Aquilano K, et al. p53 orchestrates the PGC-1alpha-mediated antioxidant response upon mild redox and metabolic imbalance. Antioxid. Redox Signal. 2013;18:386–399. doi: 10.1089/ars.2012.4615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Dhara A, et al. PGC1 alpha coactivates ERG fusion to drive antioxidant target genes under metabolic stress. Commun. Biol. 2022;5:416. doi: 10.1038/s42003-022-03385-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Garcia-Quintans N, et al. Oxidative stress induces loss of pericyte coverage and vascular instability in PGC-1alpha-deficient mice. Angiogenesis. 2016;19:217–228. doi: 10.1007/s10456-016-9502-0. [DOI] [PubMed] [Google Scholar]
- 190.Sanchez-Ramos C, et al. PGC-1alpha Downregulation in Steatotic Liver Enhances Ischemia-Reperfusion Injury and Impairs Ischemic Preconditioning. Antioxid. Redox Signal. 2017;27:1332–1346. doi: 10.1089/ars.2016.6836. [DOI] [PubMed] [Google Scholar]
- 191.Cherry AD, et al. Peroxisome proliferator-activated receptor gamma co-activator 1-alpha as a critical co-activator of the murine hepatic oxidative stress response and mitochondrial biogenesis in Staphylococcus. aureus sepsis J. Biol. Chem. 2014;289:41–52. doi: 10.1074/jbc.M113.512483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Marmolino D, et al. PGC-1alpha down-regulation affects the antioxidant response in Friedreich’s ataxia. PLoS One. 2010;5:e10025. doi: 10.1371/journal.pone.0010025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Medzhitov R. The spectrum of inflammatory responses. Science. 2021;374:1070–1075. doi: 10.1126/science.abi5200. [DOI] [PubMed] [Google Scholar]
- 194.Palomer X, et al. TNF-alpha reduces PGC-1alpha expression through NF-kappaB and p38 MAPK leading to increased glucose oxidation in a human cardiac cell model. Cardiovasc. Res. 2009;81:703–712. doi: 10.1093/cvr/cvn327. [DOI] [PubMed] [Google Scholar]
- 195.Gao F, et al. Atorvastatin attenuates TNF-alpha-induced increase of glucose oxidation through PGC-1alpha upregulation in cardiomyocytes. J. Cardiovasc. Pharmacol. 2012;59:500–506. doi: 10.1097/FJC.0b013e31824c853c. [DOI] [PubMed] [Google Scholar]
- 196.Rabinovich-Nikitin, I. et al. NF-kappaB p65 Attenuates Cardiomyocyte PGC-1alpha Expression in Hypoxia. Cells. 11, 1722 (2022). [DOI] [PMC free article] [PubMed]
- 197.Kim MS, et al. Tumor necrosis factor and interleukin 1 decrease RXRalpha, PPARalpha, PPARgamma, LXRalpha, and the coactivators SRC-1, PGC-1alpha, and PGC-1beta in liver cells. Metabolism. 2007;56:267–279. doi: 10.1016/j.metabol.2006.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Li J, et al. Tumour necrosis factor-alpha promotes liver ischaemia-reperfusion injury through the PGC-1alpha/Mfn2 pathway. J. Cell Mol. Med. 2014;18:1863–1873. doi: 10.1111/jcmm.12320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Tran M, et al. PGC-1alpha promotes recovery after acute kidney injury during systemic inflammation in mice. J. Clin. Invest. 2011;121:4003–4014. doi: 10.1172/JCI58662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Chen XH, et al. TNF-alpha induces mitochondrial dysfunction in 3T3-L1 adipocytes. Mol. Cell Endocrinol. 2010;328:63–69. doi: 10.1016/j.mce.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 201.Kalogeris TJ, et al. Adenosine prevents TNFalpha-induced decrease in endothelial mitochondrial mass via activation of eNOS-PGC-1alpha regulatory axis. PLoS One. 2014;9:e98459. doi: 10.1371/journal.pone.0098459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.De Nuccio C, et al. Peroxisome proliferator activated receptor-gamma agonists protect oligodendrocyte progenitors against tumor necrosis factor-alpha-induced damage: Effects on mitochondrial functions and differentiation. Exp. Neurol. 2015;271:506–514. doi: 10.1016/j.expneurol.2015.07.014. [DOI] [PubMed] [Google Scholar]
- 203.Alvarez-Guardia D, et al. The p65 subunit of NF-kappaB binds to PGC-1alpha, linking inflammation and metabolic disturbances in cardiac cells. Cardiovasc. Res. 2010;87:449–458. doi: 10.1093/cvr/cvq080. [DOI] [PubMed] [Google Scholar]
- 204.Huang Q, et al. Pgc-1alpha Promotes Phosphorylation, Inflammation, and Apoptosis in H9c2 Cells During the Early Stage of Lipopolysaccharide Induction. Inflammation. 2021;44:1771–1781. doi: 10.1007/s10753-021-01453-8. [DOI] [PubMed] [Google Scholar]
- 205.Wang L, et al. Isoliquiritigenin alleviates LPS/ D-GalN-induced acute liver failure by activating the PGC-1alpha/ Nrf2 pathway to reduce oxidative stress and inflammatory response. Int. Immunopharmacol. 2021;100:108159. doi: 10.1016/j.intimp.2021.108159. [DOI] [PubMed] [Google Scholar]
- 206.Xie S, et al. Activation of GPR39 with TC-G 1008 attenuates neuroinflammation via SIRT1/PGC-1alpha/Nrf2 pathway post-neonatal hypoxic-ischemic injury in rats. J. Neuroinflammation. 2021;18:226. doi: 10.1186/s12974-021-02289-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Kim, S. Y. et al. Tartary Buckwheat Extract Attenuated the Obesity-Induced Inflammation and Increased Muscle PGC-1a/SIRT1 Expression in High Fat Diet-Induced Obese Rats. Nutrients. 11, (2019). [DOI] [PMC free article] [PubMed]
- 208.Fu J, et al. Structural Mechanisms of NLRP3 Inflammasome Assembly and Activation. Annu. Rev. Immunol. 2023;41:301–316. doi: 10.1146/annurev-immunol-081022-021207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Nam BY, et al. PGC-1alpha inhibits the NLRP3 inflammasome via preserving mitochondrial viability to protect kidney fibrosis. Cell Death Dis. 2022;13:31. doi: 10.1038/s41419-021-04480-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Eisele PS, et al. The peroxisome proliferator-activated receptor gamma coactivator 1alpha/beta (PGC-1) coactivators repress the transcriptional activity of NF-kappaB in skeletal muscle cells. J. Biol. Chem. 2013;288:2246–2260. doi: 10.1074/jbc.M112.375253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Eisele PS, et al. The PGC-1 coactivators promote an anti-inflammatory environment in skeletal muscle in vivo. Biochem Biophys. Res Commun. 2015;464:692–697. doi: 10.1016/j.bbrc.2015.06.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Wang M, et al. Protein misfolding in the endoplasmic reticulum as a conduit to human disease. Nature. 2016;529:326–335. doi: 10.1038/nature17041. [DOI] [PubMed] [Google Scholar]
- 213.Walter P, et al. The unfolded protein response: from stress pathway to homeostatic regulation. Science. 2011;334:1081–1086. doi: 10.1126/science.1209038. [DOI] [PubMed] [Google Scholar]
- 214.Wu J, et al. The unfolded protein response mediates adaptation to exercise in skeletal muscle through a PGC-1alpha/ATF6alpha complex. Cell Metab. 2011;13:160–169. doi: 10.1016/j.cmet.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Misra J, et al. Transcriptional cross talk between orphan nuclear receptor ERRgamma and transmembrane transcription factor ATF6alpha coordinates endoplasmic reticulum stress response. Nucleic Acids Res. 2013;41:6960–6974. doi: 10.1093/nar/gkt429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Pan H, et al. Peroxisome proliferator-activated receptor gamma coactivator-1alpha (PGC-1alpha) overexpression alleviates endoplasmic reticulum stress after acute kidney injury. Ren. Fail. 2022;44:358–367. doi: 10.1080/0886022X.2022.2035764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Chen X, et al. Endoplasmic Reticulum Stress-Induced CHOP Inhibits PGC-1alpha and Causes Mitochondrial Dysfunction in Diabetic Embryopathy. Toxicol. Sci. 2017;158:275–285. doi: 10.1093/toxsci/kfx096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Montori-Grau M, et al. Endoplasmic reticulum stress downregulates PGC-1alpha in skeletal muscle through ATF4 and an mTOR-mediated reduction of CRTC2. Cell Commun. Signal. 2022;20:53. doi: 10.1186/s12964-022-00865-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Han HS, et al. Regulation of glucose metabolism from a liver-centric perspective. Exp. Mol. Med. 2016;48:e218. doi: 10.1038/emm.2015.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Shaw RJ, et al. The kinase LKB1 mediates glucose homeostasis in liver and therapeutic effects of metformin. Science. 2005;310:1642–1646. doi: 10.1126/science.1120781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Miao J, et al. Functional inhibitory cross-talk between constitutive androstane receptor and hepatic nuclear factor-4 in hepatic lipid/glucose metabolism is mediated by competition for binding to the DR1 motif and to the common coactivators, GRIP-1 and PGC-1alpha. J. Biol. Chem. 2006;281:14537–14546. doi: 10.1074/jbc.M510713200. [DOI] [PubMed] [Google Scholar]
- 222.Koo SH, et al. The CREB coactivator TORC2 is a key regulator of fasting glucose metabolism. Nature. 2005;437:1109–1111. doi: 10.1038/nature03967. [DOI] [PubMed] [Google Scholar]
- 223.Mitra R, et al. The transcriptional coactivators, PGC-1alpha and beta, cooperate to maintain cardiac mitochondrial function during the early stages of insulin resistance. J. Mol. Cell Cardiol. 2012;52:701–710. doi: 10.1016/j.yjmcc.2011.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Gu L, et al. Spexin alleviates insulin resistance and inhibits hepatic gluconeogenesis via the FoxO1/PGC-1alpha pathway in high-fat-diet-induced rats and insulin resistant cells. Int J. Biol. Sci. 2019;15:2815–2829. doi: 10.7150/ijbs.31781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Kleiner S, et al. Development of insulin resistance in mice lacking PGC-1alpha in adipose tissues. Proc. Natl Acad. Sci. Usa. 2012;109:9635–9640. doi: 10.1073/pnas.1207287109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Michael LF, et al. Restoration of insulin-sensitive glucose transporter (GLUT4) gene expression in muscle cells by the transcriptional coactivator PGC-1. Proc. Natl Acad. Sci. Usa. 2001;98:3820–3825. doi: 10.1073/pnas.061035098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Benton CR, et al. Modest PGC-1alpha overexpression in muscle in vivo is sufficient to increase insulin sensitivity and palmitate oxidation in subsarcolemmal, not intermyofibrillar, mitochondria. J. Biol. Chem. 2008;283:4228–4240. doi: 10.1074/jbc.M704332200. [DOI] [PubMed] [Google Scholar]
- 228.Wende AR, et al. A role for the transcriptional coactivator PGC-1alpha in muscle refueling. J. Biol. Chem. 2007;282:36642–36651. doi: 10.1074/jbc.M707006200. [DOI] [PubMed] [Google Scholar]
- 229.Mormeneo E, et al. PGC-1alpha induces mitochondrial and myokine transcriptional programs and lipid droplet and glycogen accumulation in cultured human skeletal muscle cells. PLoS One. 2012;7:e29985. doi: 10.1371/journal.pone.0029985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Lin J, et al. PGC-1beta in the regulation of hepatic glucose and energy metabolism. J. Biol. Chem. 2003;278:30843–30848. doi: 10.1074/jbc.M303643200. [DOI] [PubMed] [Google Scholar]
- 231.Nagai Y, et al. The role of peroxisome proliferator-activated receptor gamma coactivator-1 beta in the pathogenesis of fructose-induced insulin resistance. Cell Metab. 2009;9:252–264. doi: 10.1016/j.cmet.2009.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Huang TY, et al. Overexpression of PGC-1alpha increases peroxisomal activity and mitochondrial fatty acid oxidation in human primary myotubes. Am. J. Physiol. Endocrinol. Metab. 2017;312:E253–E263. doi: 10.1152/ajpendo.00331.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Summermatter S, et al. Peroxisome proliferator-activated receptor gamma coactivator 1alpha (PGC-1alpha) promotes skeletal muscle lipid refueling in vivo by activating de novo lipogenesis and the pentose phosphate pathway. J. Biol. Chem. 2010;285:32793–32800. doi: 10.1074/jbc.M110.145995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Uldry M, et al. Complementary action of the PGC-1 coactivators in mitochondrial biogenesis and brown fat differentiation. Cell Metab. 2006;3:333–341. doi: 10.1016/j.cmet.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 235.Fisher FM, et al. FGF21 regulates PGC-1alpha and browning of white adipose tissues in adaptive thermogenesis. Genes Dev. 2012;26:271–281. doi: 10.1101/gad.177857.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Carino A, et al. Gpbar1 agonism promotes a Pgc-1alpha-dependent browning of white adipose tissue and energy expenditure and reverses diet-induced steatohepatitis in mice. Sci. Rep. 2017;7:13689. doi: 10.1038/s41598-017-13102-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Chambers KT, et al. PGC-1beta and ChREBP partner to cooperatively regulate hepatic lipogenesis in a glucose concentration-dependent manner. Mol. Metab. 2013;2:194–204. doi: 10.1016/j.molmet.2013.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Sonoda J, et al. PGC-1beta controls mitochondrial metabolism to modulate circadian activity, adaptive thermogenesis, and hepatic steatosis. Proc. Natl Acad. Sci. Usa. 2007;104:5223–5228. doi: 10.1073/pnas.0611623104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Bellafante E, et al. Hepatic-specific activation of peroxisome proliferator-activated receptor gamma coactivator-1beta protects against steatohepatitis. Hepatology. 2013;57:1343–1356. doi: 10.1002/hep.26222. [DOI] [PubMed] [Google Scholar]
- 240.Chambers KT, et al. Liver-specific PGC-1beta deficiency leads to impaired mitochondrial function and lipogenic response to fasting-refeeding. PLoS One. 2012;7:e52645. doi: 10.1371/journal.pone.0052645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Hatazawa Y, et al. PGC-1alpha-mediated branched-chain amino acid metabolism in the skeletal muscle. PLoS One. 2014;9:e91006. doi: 10.1371/journal.pone.0091006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Sjogren RJO, et al. Branched-chain amino acid metabolism is regulated by ERRalpha in primary human myotubes and is further impaired by glucose loading in type 2 diabetes. Diabetologia. 2021;64:2077–2091. doi: 10.1007/s00125-021-05481-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Jang C, et al. A branched-chain amino acid metabolite drives vascular fatty acid transport and causes insulin resistance. Nat. Med. 2016;22:421–426. doi: 10.1038/nm.4057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Hatazawa Y, et al. PGC-1alpha regulates alanine metabolism in muscle cells. PLoS One. 2018;13:e0190904. doi: 10.1371/journal.pone.0190904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Tadaishi M, et al. Effect of exercise intensity and AICAR on isoform-specific expressions of murine skeletal muscle PGC-1alpha mRNA: a role of beta(2)-adrenergic receptor activation. Am. J. Physiol. Endocrinol. Metab. 2011;300:E341–E349. doi: 10.1152/ajpendo.00400.2010. [DOI] [PubMed] [Google Scholar]
- 246.Silvennoinen, M. et al. PGC-1 isoforms and their target genes are expressed differently in human skeletal muscle following resistance and endurance exercise. Physiol Rep. 3, 1722 (2015). [DOI] [PMC free article] [PubMed]
- 247.Norrbom J, et al. Alternative splice variant PGC-1alpha-b is strongly induced by exercise in human skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 2011;301:E1092–E1098. doi: 10.1152/ajpendo.00119.2011. [DOI] [PubMed] [Google Scholar]
- 248.Gidlund EK, et al. Rapidly elevated levels of PGC-1alpha-b protein in human skeletal muscle after exercise: exploring regulatory factors in a randomized controlled trial. J. Appl Physiol. (1985). 2015;119:374–384. doi: 10.1152/japplphysiol.01000.2014. [DOI] [PubMed] [Google Scholar]
- 249.Brandt N, et al. PGC-1alpha and exercise intensity dependent adaptations in mouse skeletal muscle. PLoS One. 2017;12:e0185993. doi: 10.1371/journal.pone.0185993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Yoshioka T, et al. Identification and characterization of an alternative promoter of the human PGC-1alpha gene. Biochem Biophys. Res Commun. 2009;381:537–543. doi: 10.1016/j.bbrc.2009.02.077. [DOI] [PubMed] [Google Scholar]
- 251.Tadaishi M, et al. Skeletal muscle-specific expression of PGC-1alpha-b, an exercise-responsive isoform, increases exercise capacity and peak oxygen uptake. PLoS One. 2011;6:e28290. doi: 10.1371/journal.pone.0028290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Zhang Y, et al. Alternative mRNA splicing produces a novel biologically active short isoform of PGC-1alpha. J. Biol. Chem. 2009;284:32813–32826. doi: 10.1074/jbc.M109.037556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Popov DV, et al. Exercise-induced expression of peroxisome proliferator-activated receptor gamma coactivator-1alpha isoforms in skeletal muscle of endurance-trained males. J. Physiol. Sci. 2014;64:317–323. doi: 10.1007/s12576-014-0321-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Wen X, et al. Effect of exercise intensity on isoform-specific expressions of NT-PGC-1 alpha mRNA in mouse skeletal muscle. Biomed. Res Int. 2014;2014:402175. doi: 10.1155/2014/402175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Chang, J. S. et al. Transcriptional coactivator NT-PGC-1alpha promotes gluconeogenic gene expression and enhances hepatic gluconeogenesis. Physiol Rep. 4, (2016). [DOI] [PMC free article] [PubMed]
- 256.Chang JS, et al. An unexpected role for the transcriptional coactivator isoform NT-PGC-1alpha in the regulation of mitochondrial respiration in brown adipocytes. J. Biol. Chem. 2017;292:9958–9966. doi: 10.1074/jbc.M117.778373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Chang JS, et al. A map of the PGC-1alpha- and NT-PGC-1alpha-regulated transcriptional network in brown adipose tissue. Sci. Rep. 2018;8:7876. doi: 10.1038/s41598-018-26244-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Liu Z, et al. N‑terminal truncated peroxisome proliferator‑activated receptor‑gamma coactivator‑1alpha alleviates phenylephrine‑induced mitochondrial dysfunction and decreases lipid droplet accumulation in neonatal rat cardiomyocytes. Mol. Med Rep. 2018;18:2142–2152. doi: 10.3892/mmr.2018.9158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Kim J, et al. NT-PGC-1alpha deficiency decreases mitochondrial FA oxidation in brown adipose tissue and alters substrate utilization in vivo. J. Lipid Res. 2018;59:1660–1670. doi: 10.1194/jlr.M085647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Kim J, et al. NT-PGC-1alpha deficiency attenuates high-fat diet-induced obesity by modulating food intake, fecal fat excretion and intestinal fat absorption. Sci. Rep. 2021;11:1323. doi: 10.1038/s41598-020-79823-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Chang JS, et al. NT-PGC-1alpha protein is sufficient to link beta3-adrenergic receptor activation to transcriptional and physiological components of adaptive thermogenesis. J. Biol. Chem. 2012;287:9100–9111. doi: 10.1074/jbc.M111.320200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Ruas JL, et al. A PGC-1alpha isoform induced by resistance training regulates skeletal muscle hypertrophy. Cell. 2012;151:1319–1331. doi: 10.1016/j.cell.2012.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Martinez-Redondo V, et al. Peroxisome Proliferator-activated Receptor gamma Coactivator-1 alpha Isoforms Selectively Regulate Multiple Splicing Events on Target Genes. J. Biol. Chem. 2016;291:15169–15184. doi: 10.1074/jbc.M115.705822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Leveille M, et al. PGC-1alpha isoforms coordinate to balance hepatic metabolism and apoptosis in inflammatory environments. Mol. Metab. 2020;34:72–84. doi: 10.1016/j.molmet.2020.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Thom R, et al. Hypoxic induction of vascular endothelial growth factor (VEGF) and angiogenesis in muscle by truncated peroxisome proliferator-activated receptor gamma coactivator (PGC)-1alpha. J. Biol. Chem. 2014;289:8810–8817. doi: 10.1074/jbc.M114.554394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Koh JH, et al. Enhancement of anaerobic glycolysis - a role of PGC-1alpha4 in resistance exercise. Nat. Commun. 2022;13:2324. doi: 10.1038/s41467-022-30056-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Nader GA, et al. Resistance exercise training modulates acute gene expression during human skeletal muscle hypertrophy. J. Appl Physiol. (1985). 2014;116:693–702. doi: 10.1152/japplphysiol.01366.2013. [DOI] [PubMed] [Google Scholar]
- 268.Ydfors M, et al. The truncated splice variants, NT-PGC-1alpha and PGC-1alpha4, increase with both endurance and resistance exercise in human skeletal muscle. Physiol. Rep. 2013;1:e00140. doi: 10.1002/phy2.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Lundberg TR, et al. Truncated splice variant PGC-1alpha4 is not associated with exercise-induced human muscle hypertrophy. Acta Physiol. (Oxf.). 2014;212:142–151. doi: 10.1111/apha.12310. [DOI] [PubMed] [Google Scholar]
- 270.Schwarz NA, et al. Effect of resistance exercise intensity on the expression of PGC-1alpha isoforms and the anabolic and catabolic signaling mediators, IGF-1 and myostatin, in human skeletal muscle. Appl Physiol. Nutr. Metab. 2016;41:856–863. doi: 10.1139/apnm-2016-0047. [DOI] [PubMed] [Google Scholar]
- 271.Felder TK, et al. Characterization of novel peroxisome proliferator-activated receptor gamma coactivator-1alpha (PGC-1alpha) isoform in human liver. J. Biol. Chem. 2011;286:42923–42936. doi: 10.1074/jbc.M111.227496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Yao W, et al. Endoplasmic reticulum stress links hepatitis C virus RNA replication to wild-type PGC-1alpha/liver-specific PGC-1alpha upregulation. J. Virol. 2014;88:8361–8374. doi: 10.1128/JVI.01202-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Soyal SM, et al. A greatly extended PPARGC1A genomic locus encodes several new brain-specific isoforms and influences Huntington disease age of onset. Hum. Mol. Genet. 2012;21:3461–3473. doi: 10.1093/hmg/dds177. [DOI] [PubMed] [Google Scholar]
- 274.Basu S, et al. Mutant p53 controls tumor metabolism and metastasis by regulating PGC-1alpha. Genes Dev. 2018;32:230–243. doi: 10.1101/gad.309062.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Prieto I, et al. Metabolic adaptations in spontaneously immortalized PGC-1alpha knock-out mouse embryonic fibroblasts increase their oncogenic potential. Redox Biol. 2020;29:101396. doi: 10.1016/j.redox.2019.101396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Chaube B, et al. AMPK maintains energy homeostasis and survival in cancer cells via regulating p38/PGC-1alpha-mediated mitochondrial biogenesis. Cell Death Discov. 2015;1:15063. doi: 10.1038/cddiscovery.2015.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Sun X, et al. Targeting PGC1alpha to wrestle cancer: a compelling therapeutic opportunity. J. Cancer Res Clin. Oncol. 2022;148:767–774. doi: 10.1007/s00432-021-03912-z. [DOI] [PubMed] [Google Scholar]
- 278.Dekker E, et al. Colorectal cancer. Lancet. 2019;394:1467–1480. doi: 10.1016/S0140-6736(19)32319-0. [DOI] [PubMed] [Google Scholar]
- 279.Yun SH, et al. Peroxisome proliferator-activated receptor gamma coactivator-1alpha is a predictor of lymph node metastasis and poor prognosis in human colorectal cancer. Ann. Diagn. Pathol. 2018;33:11–16. doi: 10.1016/j.anndiagpath.2017.11.007. [DOI] [PubMed] [Google Scholar]
- 280.Alonso-Molero J, et al. Alterations in PGC1alpha expression levels are involved in colorectal cancer risk: a qualitative systematic review. BMC Cancer. 2017;17:731. doi: 10.1186/s12885-017-3725-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Boughanem, H. et al. The Expression/Methylation Profile of Adipogenic and Inflammatory Transcription Factors in Adipose Tissue Are Linked to Obesity-Related Colorectal Cancer. Cancers (Basel). 11, (2019). [DOI] [PMC free article] [PubMed]
- 282.Chen W, et al. PGC-1alpha promotes colorectal carcinoma metastasis through regulating ABCA1 transcription. Oncogene. 2023;42:2456–2470. doi: 10.1038/s41388-023-02762-y. [DOI] [PubMed] [Google Scholar]
- 283.Cho, J. G. et al. PGC-1alpha Regulates Cell Proliferation, Migration, and Invasion by Modulating Leucyl-tRNA Synthetase 1 Expression in Human Colorectal Cancer Cells. Cancers (Basel). 15, (2022). [DOI] [PMC free article] [PubMed]
- 284.Yun SH, et al. PGC-1alpha Regulates Cell Proliferation and Invasion via AKT/GSK-3beta/beta-catenin Pathway in Human Colorectal Cancer SW620 and SW480 Cells. Anticancer Res. 2020;40:653–664. doi: 10.21873/anticanres.13995. [DOI] [PubMed] [Google Scholar]
- 285.Paku M, et al. SIRT3-Mediated SOD2 and PGC-1alpha Contribute to Chemoresistance in Colorectal Cancer Cells. Ann. Surg. Oncol. 2021;28:4720–4732. doi: 10.1245/s10434-020-09373-x. [DOI] [PubMed] [Google Scholar]
- 286.Biserova, K. et al. Cancer Stem Cells: Significance in Origin, Pathogenesis and Treatment of Glioblastoma. Cells. 10, (2021). [DOI] [PMC free article] [PubMed]
- 287.Liu S, et al. Lactate promotes metastasis of normoxic colorectal cancer stem cells through PGC-1alpha-mediated oxidative phosphorylation. Cell Death Dis. 2022;13:651. doi: 10.1038/s41419-022-05111-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Yun CW, et al. Hypoxia-induced PGC-1alpha Regulates Mitochondrial Function and Tumorigenesis of Colorectal Cancer Cells. Anticancer Res. 2019;39:4865–4876. doi: 10.21873/anticanres.13672. [DOI] [PubMed] [Google Scholar]
- 289.Yun, C. W. et al. PGC-1alpha Controls Mitochondrial Biogenesis in Drug-Resistant Colorectal Cancer Cells by Regulating Endoplasmic Reticulum Stress. Int. J. Mol. Sci. 20, (2019). [DOI] [PMC free article] [PubMed]
- 290.Fisher KW, et al. AMPK Promotes Aberrant PGC1beta Expression To Support Human Colon Tumor Cell Survival. Mol. Cell Biol. 2015;35:3866–3879. doi: 10.1128/MCB.00528-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.McCall JL, et al. KSR1 and EPHB4 Regulate Myc and PGC1beta To Promote Survival of Human Colon Tumors. Mol. Cell Biol. 2016;36:2246–2261. doi: 10.1128/MCB.00087-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Bellafante E, et al. PGC-1beta promotes enterocyte lifespan and tumorigenesis in the intestine. Proc. Natl Acad. Sci. Usa. 2014;111:E4523–E4531. doi: 10.1073/pnas.1415279111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Yang JD, et al. A global view of hepatocellular carcinoma: trends, risk, prevention and management. Nat. Rev. Gastroenterol. Hepatol. 2019;16:589–604. doi: 10.1038/s41575-019-0186-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Yang T, et al. OCTN2 enhances PGC-1alpha-mediated fatty acid oxidation and OXPHOS to support stemness in hepatocellular carcinoma. Metabolism. 2023;147:155628. doi: 10.1016/j.metabol.2023.155628. [DOI] [PubMed] [Google Scholar]
- 295.Kumar A, et al. Sestrin2 facilitates glutamine-dependent transcription of PGC-1alpha and survival of liver cancer cells under glucose limitation. FEBS J. 2018;285:1326–1345. doi: 10.1111/febs.14406. [DOI] [PubMed] [Google Scholar]
- 296.Tohme S, et al. Hypoxia mediates mitochondrial biogenesis in hepatocellular carcinoma to promote tumor growth through HMGB1 and TLR9 interaction. Hepatology. 2017;66:182–197. doi: 10.1002/hep.29184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Piccinin E, et al. Hepatic peroxisome proliferator-activated receptor gamma coactivator 1beta drives mitochondrial and anabolic signatures that contribute to hepatocellular carcinoma progression in mice. Hepatology. 2018;67:884–898. doi: 10.1002/hep.29484. [DOI] [PubMed] [Google Scholar]
- 298.Xu J, et al. UBQLN1 mediates sorafenib resistance through regulating mitochondrial biogenesis and ROS homeostasis by targeting PGC1beta in hepatocellular carcinoma. Signal Transduct. Target Ther. 2021;6:190. doi: 10.1038/s41392-021-00594-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Li Y, et al. Yin Yang 1 facilitates hepatocellular carcinoma cell lipid metabolism and tumor progression by inhibiting PGC-1beta-induced fatty acid oxidation. Theranostics. 2019;9:7599–7615. doi: 10.7150/thno.34931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Sung H, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 301.Klimcakova E, et al. PGC-1alpha promotes the growth of ErbB2/Neu-induced mammary tumors by regulating nutrient supply. Cancer Res. 2012;72:1538–1546. doi: 10.1158/0008-5472.CAN-11-2967. [DOI] [PubMed] [Google Scholar]
- 302.Metallo CM, et al. Reductive glutamine metabolism by IDH1 mediates lipogenesis under hypoxia. Nature. 2011;481:380–384. doi: 10.1038/nature10602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.McGuirk S, et al. PGC-1alpha supports glutamine metabolism in breast cancer. Cancer Metab. 2013;1:22. doi: 10.1186/2049-3002-1-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Andrzejewski S, et al. PGC-1alpha Promotes Breast Cancer Metastasis and Confers Bioenergetic Flexibility against Metabolic Drugs. Cell Metab. 2017;26:778–787.e775. doi: 10.1016/j.cmet.2017.09.006. [DOI] [PubMed] [Google Scholar]
- 305.LeBleu VS, et al. PGC-1alpha mediates mitochondrial biogenesis and oxidative phosphorylation in cancer cells to promote metastasis. Nat. Cell Biol. 2014;16:992–1003. doi: 10.1038/ncb3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Ansari MI, et al. Bisphenol A exposure induces metastatic aggression in low metastatic MCF-7 cells via PGC-1alpha mediated mitochondrial biogenesis and epithelial-mesenchymal plasticity. Life Sci. 2022;302:120649. doi: 10.1016/j.lfs.2022.120649. [DOI] [PubMed] [Google Scholar]
- 307.Audet-Walsh E, et al. The PGC-1alpha/ERRalpha Axis Represses One-Carbon Metabolism and Promotes Sensitivity to Anti-folate Therapy in Breast Cancer. Cell Rep. 2016;14:920–931. doi: 10.1016/j.celrep.2015.12.086. [DOI] [PubMed] [Google Scholar]
- 308.Lou C, et al. MiR-485-3p and miR-485-5p suppress breast cancer cell metastasis by inhibiting PGC-1alpha expression. Cell Death Dis. 2016;7:e2159. doi: 10.1038/cddis.2016.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Zhou, H. et al. miR‑382 inhibits breast cancer progression and metastasis by affecting the M2 polarization of tumor‑associated macrophages by targeting PGC‑1alpha. Int. J. Oncol. 61, (2022). [DOI] [PMC free article] [PubMed]
- 310.Victorino VJ, et al. PGC-1beta regulates HER2-overexpressing breast cancer cells proliferation by metabolic and redox pathways. Tumour Biol. 2016;37:6035–6044. doi: 10.1007/s13277-015-4449-0. [DOI] [PubMed] [Google Scholar]
- 311.Cao J, et al. PGC-1beta cooperating with FOXA2 inhibits proliferation and migration of breast cancer cells. Cancer Cell Int. 2019;19:93. doi: 10.1186/s12935-019-0810-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Chen X, et al. PGC1beta Regulates Breast Tumor Growth and Metastasis by SREBP1-Mediated HKDC1 Expression. Front Oncol. 2019;9:290. doi: 10.3389/fonc.2019.00290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Deblois G, et al. Transcriptional control of the ERBB2 amplicon by ERRalpha and PGC-1beta promotes mammary gland tumorigenesis. Cancer Res. 2010;70:10277–10287. doi: 10.1158/0008-5472.CAN-10-2840. [DOI] [PubMed] [Google Scholar]
- 314.Wang X, et al. miR-22-3p/PGC1beta Suppresses Breast Cancer Cell Tumorigenesis via PPARgamma. PPAR Res. 2021;2021:6661828. doi: 10.1155/2021/6661828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Eichner LJ, et al. miR-378(*) mediates metabolic shift in breast cancer cells via the PGC-1beta/ERRgamma transcriptional pathway. Cell Metab. 2010;12:352–361. doi: 10.1016/j.cmet.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 316.Zhu JW, et al. Potential clinical utility of liquid biopsies in ovarian cancer. Mol. Cancer. 2022;21:114. doi: 10.1186/s12943-022-01588-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Ghilardi C, et al. PGC1alpha/beta Expression Predicts Therapeutic Response to Oxidative Phosphorylation Inhibition in Ovarian Cancer. Cancer Res. 2022;82:1423–1434. doi: 10.1158/0008-5472.CAN-21-1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Guo T, et al. GCN-5/PGC-1alpha signaling is activated and associated with metabolism in cyclin E1-driven ovarian cancer. Aging (Albany N. Y.). 2019;11:4890–4899. doi: 10.18632/aging.102082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Gentric G, et al. PML-Regulated Mitochondrial Metabolism Enhances Chemosensitivity in Human Ovarian Cancers. Cell Metab. 2019;29:156–173 e110. doi: 10.1016/j.cmet.2018.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Guo T, et al. PGC-1alpha inhibits polyamine metabolism in Cyclin E1-driven ovarian cancer. Cancer Med. 2019;8:7754–7761. doi: 10.1002/cam4.2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Urick ME, et al. Clinical actionability of molecular targets in endometrial cancer. Nat. Rev. Cancer. 2019;19:510–521. doi: 10.1038/s41568-019-0177-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Yoriki K, et al. Estrogen-related receptor alpha induces epithelial-mesenchymal transition through cancer-stromal interactions in endometrial cancer. Sci. Rep. 2019;9:6697. doi: 10.1038/s41598-019-43261-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Ren Z, et al. The effects of PGC-1alpha on the proliferation and energy metabolism of malignant endometrial cancer cells. Onco Targets Ther. 2015;8:769–774. doi: 10.2147/OTT.S79960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Yang H, et al. Synergism between PGC-1alpha and estrogen in the survival of endometrial cancer cells via the mitochondrial pathway. Onco Targets Ther. 2016;9:3963–3973. doi: 10.2147/OTT.S103482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Yang H, et al. Knockdown of peroxisome proliferator-activated receptor gamma coactivator-1 alpha increased apoptosis of human endometrial cancer HEC-1A cells. Onco Targets Ther. 2016;9:5329–5338. doi: 10.2147/OTT.S102816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Skudalski L, et al. Melanoma: An update on systemic therapies. J. Am. Acad. Dermatol. 2022;86:515–524. doi: 10.1016/j.jaad.2021.09.075. [DOI] [PubMed] [Google Scholar]
- 327.Vazquez F, et al. PGC1alpha expression defines a subset of human melanoma tumors with increased mitochondrial capacity and resistance to oxidative stress. Cancer Cell. 2013;23:287–301. doi: 10.1016/j.ccr.2012.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Luo C, et al. A PGC1alpha-mediated transcriptional axis suppresses melanoma metastasis. Nature. 2016;537:422–426. doi: 10.1038/nature19347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Gelato KA, et al. Super-enhancers define a proliferative PGC-1alpha-expressing melanoma subgroup sensitive to BET inhibition. Oncogene. 2018;37:512–521. doi: 10.1038/onc.2017.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Kumar PR, et al. PGC-1alpha induced mitochondrial biogenesis in stromal cells underpins mitochondrial transfer to melanoma. Br. J. Cancer. 2022;127:69–78. doi: 10.1038/s41416-022-01783-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Fujimura T, et al. BRAF kinase inhibitors for treatment of melanoma: developments from early-stage animal studies to Phase II clinical trials. Expert Opin. Investig. Drugs. 2019;28:143–148. doi: 10.1080/13543784.2019.1558442. [DOI] [PubMed] [Google Scholar]
- 332.Ferretta A, et al. New insight into the role of metabolic reprogramming in melanoma cells harboring BRAF mutations. Biochim Biophys. Acta. 2016;1863:2710–2718. doi: 10.1016/j.bbamcr.2016.08.007. [DOI] [PubMed] [Google Scholar]
- 333.Shoag J, et al. PGC-1 coactivators regulate MITF and the tanning response. Mol. Cell. 2013;49:145–157. doi: 10.1016/j.molcel.2012.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Laurin KM, et al. Low expression of PGC-1beta and other mitochondrial biogenesis modulators in melanoma is associated with growth arrest and the induction of an immunosuppressive gene expression program dependent on MEK and IRF-1. Cancer Lett. 2022;541:215738. doi: 10.1016/j.canlet.2022.215738. [DOI] [PubMed] [Google Scholar]
- 335.Siegel RL, et al. Cancer Statistics, 2021. CA Cancer J. Clin. 2021;71:7–33. doi: 10.3322/caac.21654. [DOI] [PubMed] [Google Scholar]
- 336.Huang X, et al. LINC00842 inactivates transcription co-regulator PGC-1alpha to promote pancreatic cancer malignancy through metabolic remodelling. Nat. Commun. 2021;12:3830. doi: 10.1038/s41467-021-23904-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Yin QH, et al. miR-373 Suppresses Cell Proliferation and Apoptosis via Regulation of SIRT1/PGC-1alpha/NRF2 Axis in Pancreatic Cancer. Cell J. 2021;23:199–210. doi: 10.22074/cellj.2021.7038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Sancho P, et al. MYC/PGC-1alpha Balance Determines the Metabolic Phenotype and Plasticity of Pancreatic Cancer Stem Cells. Cell Metab. 2015;22:590–605. doi: 10.1016/j.cmet.2015.08.015. [DOI] [PubMed] [Google Scholar]
- 339.Nguyen-Nielsen M, et al. Diagnostic and Therapeutic Strategies for Prostate Cancer. Semin. Nucl. Med. 2016;46:484–490. doi: 10.1053/j.semnuclmed.2016.07.002. [DOI] [PubMed] [Google Scholar]
- 340.Siddappa M, et al. Identification of transcription factor co-regulators that drive prostate cancer progression. Sci. Rep. 2020;10:20332. doi: 10.1038/s41598-020-77055-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Valcarcel-Jimenez L, et al. PGC1alpha Suppresses Prostate Cancer Cell Invasion through ERRalpha Transcriptional Control. Cancer Res. 2019;79:6153–6165. doi: 10.1158/0008-5472.CAN-19-1231. [DOI] [PubMed] [Google Scholar]
- 342.Torrano V, et al. The metabolic co-regulator PGC1alpha suppresses prostate cancer metastasis. Nat. Cell Biol. 2016;18:645–656. doi: 10.1038/ncb3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Kaminski L, et al. PGC1alpha Inhibits Polyamine Synthesis to Suppress Prostate Cancer Aggressiveness. Cancer Res. 2019;79:3268–3280. doi: 10.1158/0008-5472.CAN-18-2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Wang H, et al. Targeting p53 pathways: mechanisms, structures, and advances in therapy. Signal Transduct. Target Ther. 2023;8:92. doi: 10.1038/s41392-023-01347-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Li J, et al. p53/PGC‑1alpha‑mediated mitochondrial dysfunction promotes PC3 prostate cancer cell apoptosis. Mol. Med Rep. 2020;22:155–164. doi: 10.3892/mmr.2020.11121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Shiota M, et al. Peroxisome proliferator-activated receptor gamma coactivator-1alpha interacts with the androgen receptor (AR) and promotes prostate cancer cell growth by activating the AR. Mol. Endocrinol. 2010;24:114–127. doi: 10.1210/me.2009-0302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Tennakoon JB, et al. Androgens regulate prostate cancer cell growth via an AMPK-PGC-1alpha-mediated metabolic switch. Oncogene. 2014;33:5251–5261. doi: 10.1038/onc.2013.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Garnier A, et al. Depressed mitochondrial transcription factors and oxidative capacity in rat failing cardiac and skeletal muscles. J. Physiol. 2003;551:491–501. doi: 10.1113/jphysiol.2003.045104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Arany Z, et al. Transcriptional coactivator PGC-1 alpha controls the energy state and contractile function of cardiac muscle. Cell Metab. 2005;1:259–271. doi: 10.1016/j.cmet.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 350.Lai L, et al. Transcriptional coactivators PGC-1alpha and PGC-lbeta control overlapping programs required for perinatal maturation of the heart. Genes Dev. 2008;22:1948–1961. doi: 10.1101/gad.1661708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Rowe GC, et al. PGC-1 coactivators in cardiac development and disease. Circ. Res. 2010;107:825–838. doi: 10.1161/CIRCRESAHA.110.223818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Rog-Zielinska EA, et al. Glucocorticoids promote structural and functional maturation of foetal cardiomyocytes: a role for PGC-1alpha. Cell Death Differ. 2015;22:1106–1116. doi: 10.1038/cdd.2014.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Greene SJ, et al. Worsening Heart Failure: Nomenclature, Epidemiology, and Future Directions: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2023;81:413–424. doi: 10.1016/j.jacc.2022.11.023. [DOI] [PubMed] [Google Scholar]
- 354.Sihag S, et al. PGC-1alpha and ERRalpha target gene downregulation is a signature of the failing human heart. J. Mol. Cell. Cardiol. 2009;46:201–212. doi: 10.1016/j.yjmcc.2008.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Garnier A, et al. Control by circulating factors of mitochondrial function and transcription cascade in heart failure: a role for endothelin-1 and angiotensin II. Circ. Heart Fail. 2009;2:342–350. doi: 10.1161/CIRCHEARTFAILURE.108.812099. [DOI] [PubMed] [Google Scholar]
- 356.Sebastiani M, et al. Induction of mitochondrial biogenesis is a maladaptive mechanism in mitochondrial cardiomyopathies. J. Am. Coll. Cardiol. 2007;50:1362–1369. doi: 10.1016/j.jacc.2007.06.035. [DOI] [PubMed] [Google Scholar]
- 357.Chen P, et al. Serum Peroxisome Proliferator-activated Receptor Gamma Coactivator-1alpha Related to Myocardial Energy Expenditure in Patients With Chronic Heart Failure. Am. J. Med. Sci. 2019;357:205–212. doi: 10.1016/j.amjms.2018.12.002. [DOI] [PubMed] [Google Scholar]
- 358.Fabregat-Andres O, et al. mRNA PGC-1alpha levels in blood samples reliably correlates with its myocardial expression: study in patients undergoing cardiac surgery. Anatol. J. Cardiol. 2016;16:622–629. doi: 10.5152/AnatolJCardiol.2015.6466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Bhat S, et al. Recruitment of RNA Polymerase II to Metabolic Gene Promoters Is Inhibited in the Failing Heart Possibly Through PGC-1alpha (Peroxisome Proliferator-Activated Receptor-gamma Coactivator-1alpha) Dysregulation. Circ. Heart Fail. 2019;12:e005529. doi: 10.1161/CIRCHEARTFAILURE.118.005529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Hu X, et al. AMP activated protein kinase-alpha2 regulates expression of estrogen-related receptor-alpha, a metabolic transcription factor related to heart failure development. Hypertension. 2011;58:696–703. doi: 10.1161/HYPERTENSIONAHA.111.174128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Arany Z, et al. Transverse aortic constriction leads to accelerated heart failure in mice lacking PPAR-gamma coactivator 1alpha. Proc. Natl Acad. Sci. Usa. 2006;103:10086–10091. doi: 10.1073/pnas.0603615103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Karkkainen O, et al. Heart specific PGC-1alpha deletion identifies metabolome of cardiac restricted metabolic heart failure. Cardiovasc. Res. 2019;115:107–118. doi: 10.1093/cvr/cvy155. [DOI] [PubMed] [Google Scholar]
- 363.Naumenko N, et al. PGC-1alpha deficiency reveals sex-specific links between cardiac energy metabolism and EC-coupling during development of heart failure in mice. Cardiovasc. Res. 2022;118:1520–1534. doi: 10.1093/cvr/cvab188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Zhuang L, et al. DYRK1B-STAT3 Drives Cardiac Hypertrophy and Heart Failure by Impairing Mitochondrial Bioenergetics. Circulation. 2022;145:829–846. doi: 10.1161/CIRCULATIONAHA.121.055727. [DOI] [PubMed] [Google Scholar]
- 365.Miao W, et al. Nr2f2 Overexpression Aggravates Ferroptosis and Mitochondrial Dysfunction by Regulating the PGC-1alpha Signaling in Diabetes-Induced Heart Failure Mice. Mediators Inflamm. 2022;2022:8373389. doi: 10.1155/2022/8373389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Karamanlidis G, et al. Promoting PGC-1alpha-driven mitochondrial biogenesis is detrimental in pressure-overloaded mouse hearts. Am. J. Physiol. Heart Circ. Physiol. 2014;307:H1307–H1316. doi: 10.1152/ajpheart.00280.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Zhu X, et al. Fine-Tuning of PGC1alpha Expression Regulates Cardiac Function and Longevity. Circ. Res. 2019;125:707–719. doi: 10.1161/CIRCRESAHA.119.315529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Nakamura M, et al. Mechanisms of physiological and pathological cardiac hypertrophy. Nat. Rev. Cardiol. 2018;15:387–407. doi: 10.1038/s41569-018-0007-y. [DOI] [PubMed] [Google Scholar]
- 369.Oka T, et al. Angiogenesis and cardiac hypertrophy: maintenance of cardiac function and causative roles in heart failure. Circ. Res. 2014;114:565–571. doi: 10.1161/CIRCRESAHA.114.300507. [DOI] [PubMed] [Google Scholar]
- 370.Li L, et al. Mitochondrial biogenesis and PGC-1alpha deacetylation by chronic treadmill exercise: differential response in cardiac and skeletal muscle. Basic Res Cardiol. 2011;106:1221–1234. doi: 10.1007/s00395-011-0213-9. [DOI] [PubMed] [Google Scholar]
- 371.Tuomainen, T. et al. PGC-1alpha4 Interacts with REST to Upregulate Neuronal Genes and Augment Energy Consumption in Developing Cardiomyocytes. Cells. 11, (2022). [DOI] [PMC free article] [PubMed]
- 372.Gomez-Arroyo J, et al. Metabolic gene remodeling and mitochondrial dysfunction in failing right ventricular hypertrophy secondary to pulmonary arterial hypertension. Circ. Heart Fail. 2013;6:136–144. doi: 10.1161/CIRCHEARTFAILURE.111.966127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Brainard RE, et al. Cardiac hypertrophy drives PGC-1alpha suppression associated with enhanced O-glycosylation. Biochim Biophys. Acta Mol. Basis Dis. 2021;1867:166080. doi: 10.1016/j.bbadis.2021.166080. [DOI] [PubMed] [Google Scholar]
- 374.Xu W, et al. The protective role of peroxisome proliferator-activated receptor gamma coactivator-1alpha in hyperthyroid cardiac hypertrophy. J. Cell. Physiol. 2012;227:3243–3253. doi: 10.1002/jcp.24015. [DOI] [PubMed] [Google Scholar]
- 375.Liu XP, et al. Peroxisome proliferator-activated receptor gamma coactivator 1 alpha protects cardiomyocytes from hypertrophy by suppressing calcineurin-nuclear factor of activated T cells c4 signaling pathway. Transl. Res. 2015;166:459–473.e453. doi: 10.1016/j.trsl.2015.06.003. [DOI] [PubMed] [Google Scholar]
- 376.Pereira RO, et al. Maintaining PGC-1alpha expression following pressure overload-induced cardiac hypertrophy preserves angiogenesis but not contractile or mitochondrial function. FASEB J. 2014;28:3691–3702. doi: 10.1096/fj.14-253823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Riehle C, et al. PGC-1beta deficiency accelerates the transition to heart failure in pressure overload hypertrophy. Circ. Res. 2011;109:783–793. doi: 10.1161/CIRCRESAHA.111.243964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Zhai, M. et al. Melatonin protects against the pathological cardiac hypertrophy induced by transverse aortic constriction through activating PGC-1beta: In vivo and in vitro studies. J. Pineal Res. 63, (2017). [DOI] [PubMed]
- 379.Hilfiker-Kleiner D, et al. Pathophysiology and epidemiology of peripartum cardiomyopathy. Nat. Rev. Cardiol. 2014;11:364–370. doi: 10.1038/nrcardio.2014.37. [DOI] [PubMed] [Google Scholar]
- 380.Dillmann WH. Diabetic Cardiomyopathy. Circ. Res. 2019;124:1160–1162. doi: 10.1161/CIRCRESAHA.118.314665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Ren J, et al. Obesity cardiomyopathy: evidence, mechanisms, and therapeutic implications. Physiol. Rev. 2021;101:1745–1807. doi: 10.1152/physrev.00030.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Jia G, et al. Insulin resistance and hyperinsulinaemia in diabetic cardiomyopathy. Nat. Rev. Endocrinol. 2016;12:144–153. doi: 10.1038/nrendo.2015.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383.Murtaza G, et al. Diabetic cardiomyopathy - A comprehensive updated review. Prog. Cardiovasc. Dis. 2019;62:315–326. doi: 10.1016/j.pcad.2019.03.003. [DOI] [PubMed] [Google Scholar]
- 384.Waldman M, et al. PARP-1 inhibition protects the diabetic heart through activation of SIRT1-PGC-1alpha axis. Exp. Cell Res. 2018;373:112–118. doi: 10.1016/j.yexcr.2018.10.003. [DOI] [PubMed] [Google Scholar]
- 385.Burkart EM, et al. Nuclear receptors PPARbeta/delta and PPARalpha direct distinct metabolic regulatory programs in the mouse heart. J. Clin. Invest. 2007;117:3930–3939. doi: 10.1172/JCI32578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Hu T, et al. PRDM16 exerts critical role in myocardial metabolism and energetics in type 2 diabetes induced cardiomyopathy. Metabolism. 2023;146:155658. doi: 10.1016/j.metabol.2023.155658. [DOI] [PubMed] [Google Scholar]
- 387.Hu N, et al. Mitochondrial aldehyde dehydrogenase obliterates insulin resistance-induced cardiac dysfunction through deacetylation of PGC-1alpha. Oncotarget. 2016;7:76398–76414. doi: 10.18632/oncotarget.11977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388.Wang SY, et al. Exercise enhances cardiac function by improving mitochondrial dysfunction and maintaining energy homoeostasis in the development of diabetic cardiomyopathy. J. Mol. Med. (Berl.). 2020;98:245–261. doi: 10.1007/s00109-019-01861-2. [DOI] [PubMed] [Google Scholar]
- 389.Honigberg MC, et al. Peripartum cardiomyopathy. BMJ. 2019;364:k5287. doi: 10.1136/bmj.k5287. [DOI] [PubMed] [Google Scholar]
- 390.Patten IS, et al. Cardiac angiogenic imbalance leads to peripartum cardiomyopathy. Nature. 2012;485:333–338. doi: 10.1038/nature11040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391.Zhang Y, et al. beta1-Adrenoceptor antibodies induce PPCM via inhibition of PGC-1alpha related pathway. Scand. Cardiovasc. J. 2021;55:160–167. doi: 10.1080/14017431.2020.1869300. [DOI] [PubMed] [Google Scholar]
- 392.Garcia MM, et al. Methyl donor deficiency induces cardiomyopathy through altered methylation/acetylation of PGC-1alpha by PRMT1 and SIRT1. J. Pathol. 2011;225:324–335. doi: 10.1002/path.2881. [DOI] [PubMed] [Google Scholar]
- 393.Huang CY, et al. PERM1 regulates genes involved in fatty acid metabolism in the heart by interacting with PPARalpha and PGC-1alpha. Sci. Rep. 2022;12:14576. doi: 10.1038/s41598-022-18885-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394.Hoes MF, et al. Pathophysiology and risk factors of peripartum cardiomyopathy. Nat. Rev. Cardiol. 2022;19:555–565. doi: 10.1038/s41569-021-00664-8. [DOI] [PubMed] [Google Scholar]
- 395.Wang S, et al. ALDH2 protects against high fat diet-induced obesity cardiomyopathy and defective autophagy: role of CaM kinase II, histone H3K9 methyltransferase SUV39H, Sirt1, and PGC-1alpha deacetylation. Int. J. Obes. (Lond.). 2018;42:1073–1087. doi: 10.1038/s41366-018-0030-4. [DOI] [PubMed] [Google Scholar]
- 396.Diop SB, et al. PGC-1/Spargel Counteracts High-Fat-Diet-Induced Obesity and Cardiac Lipotoxicity Downstream of TOR and Brummer ATGL Lipase. Cell Rep. 2015;10:1572–1584. doi: 10.1016/j.celrep.2015.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 397.He SL, et al. Mitochondrial-related gene expression profiles suggest an important role of PGC-1alpha in the compensatory mechanism of endemic dilated cardiomyopathy. Exp. Cell Res. 2013;319:2604–2616. doi: 10.1016/j.yexcr.2013.07.018. [DOI] [PubMed] [Google Scholar]
- 398.Jiang S, et al. Relationship Between Myocardial Injury and Expression of PGC-1alpha and Its Coactivators in Chronic Keshan Disease. Curr. Med Sci. 2022;42:85–92. doi: 10.1007/s11596-021-2454-7. [DOI] [PubMed] [Google Scholar]
- 399.Ahmad S, et al. Effects of ageing on pro-arrhythmic ventricular phenotypes in incrementally paced murine Pgc-1beta (-/-) hearts. Pflug. Arch. 2017;469:1579–1590. doi: 10.1007/s00424-017-2054-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400.Valli H, et al. Age-dependent atrial arrhythmic phenotype secondary to mitochondrial dysfunction in Pgc-1beta deficient murine hearts. Mech. Ageing Dev. 2017;167:30–45. doi: 10.1016/j.mad.2017.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401.Ahmad S, et al. Ventricular pro-arrhythmic phenotype, arrhythmic substrate, ageing and mitochondrial dysfunction in peroxisome proliferator activated receptor-gamma coactivator-1beta deficient (Pgc-1beta(-/-)) murine hearts. Mech. Ageing Dev. 2018;173:92–103. doi: 10.1016/j.mad.2018.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402.Ahmad S, et al. Reduced cardiomyocyte Na(+) current in the age-dependent murine Pgc-1beta(-/-) model of ventricular arrhythmia. J. Cell. Physiol. 2019;234:3921–3932. doi: 10.1002/jcp.27183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 403.Chadda, K. R. et al. Gene and Protein Expression Profile of Selected Molecular Targets Mediating Electrophysiological Function in Pgc-1alpha Deficient Murine Atria. Int. J. Mol. Sci. 19, (2018). [DOI] [PMC free article] [PubMed]
- 404.Libby P, et al. Progress and challenges in translating the biology of atherosclerosis. Nature. 2011;473:317–325. doi: 10.1038/nature10146. [DOI] [PubMed] [Google Scholar]
- 405.Zhang Y, et al. Association between PPARGC1A gene polymorphisms and coronary artery disease in a Chinese population. Clin. Exp. Pharm. Physiol. 2008;35:1172–1177. doi: 10.1111/j.1440-1681.2008.04988.x. [DOI] [PubMed] [Google Scholar]
- 406.Forstermann U, et al. Roles of Vascular Oxidative Stress and Nitric Oxide in the Pathogenesis of Atherosclerosis. Circ. Res. 2017;120:713–735. doi: 10.1161/CIRCRESAHA.116.309326. [DOI] [PubMed] [Google Scholar]
- 407.Kim HJ, et al. Effects of PGC-1alpha on TNF-alpha-induced MCP-1 and VCAM-1 expression and NF-kappaB activation in human aortic smooth muscle and endothelial cells. Antioxid. Redox Signal. 2007;9:301–307. doi: 10.1089/ars.2006.1456. [DOI] [PubMed] [Google Scholar]
- 408.Won JC, et al. Peroxisome proliferator-activated receptor-gamma coactivator 1-alpha overexpression prevents endothelial apoptosis by increasing ATP/ADP translocase activity. Arterioscler. Thromb. Vasc. Biol. 2010;30:290–297. doi: 10.1161/ATVBAHA.109.198721. [DOI] [PubMed] [Google Scholar]
- 409.Zhao, Q. et al. PGC-1alpha limits angiotensin II-induced rat vascular smooth muscle cells proliferation via attenuating NOX1-mediated generation of reactive oxygen species. Biosci. Rep. 35, (2015). [DOI] [PMC free article] [PubMed]
- 410.Sun, H. et al. C1q/TNF-Related Protein-9 Ameliorates Ox-LDL-Induced Endothelial Dysfunction via PGC-1alpha/AMPK-Mediated Antioxidant Enzyme Induction. Int. J. Mol. Sci. 18, (2017). [DOI] [PMC free article] [PubMed]
- 411.Grootaert MOJ, et al. Vascular smooth muscle cell death, autophagy and senescence in atherosclerosis. Cardiovasc. Res. 2018;114:622–634. doi: 10.1093/cvr/cvy007. [DOI] [PubMed] [Google Scholar]
- 412.Qu A, et al. PGC-1alpha attenuates neointimal formation via inhibition of vascular smooth muscle cell migration in the injured rat carotid artery. Am. J. Physiol. Cell Physiol. 2009;297:C645–C653. doi: 10.1152/ajpcell.00469.2008. [DOI] [PubMed] [Google Scholar]
- 413.Jiang X, et al. 17beta-estradiol inhibits oleic acid-induced rat VSMC proliferation and migration by restoring PGC-1alpha expression. Mol. Cell Endocrinol. 2010;315:74–80. doi: 10.1016/j.mce.2009.09.018. [DOI] [PubMed] [Google Scholar]
- 414.Chong H, et al. The PGC-1alpha/NRF1/miR-378a axis protects vascular smooth muscle cells from FFA-induced proliferation, migration and inflammation in atherosclerosis. Atherosclerosis. 2020;297:136–145. doi: 10.1016/j.atherosclerosis.2020.02.001. [DOI] [PubMed] [Google Scholar]
- 415.Zhang Y, et al. PGC-1alpha inhibits oleic acid induced proliferation and migration of rat vascular smooth muscle cells. PLoS One. 2007;2:e1137. doi: 10.1371/journal.pone.0001137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 416.Zhu L, et al. PGC-1alpha is a key regulator of glucose-induced proliferation and migration in vascular smooth muscle cells. PLoS One. 2009;4:e4182. doi: 10.1371/journal.pone.0004182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 417.Gan X, et al. Plin5 inhibits proliferation and migration of vascular smooth muscle cell through interacting with PGC-1alpha following vascular injury. Bioengineered. 2022;13:10665–10678. doi: 10.1080/21655979.2022.2065762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 418.Wang D, et al. Targeting Foam Cell Formation in Atherosclerosis: Therapeutic Potential of Natural Products. Pharmacol. Rev. 2019;71:596–670. doi: 10.1124/pr.118.017178. [DOI] [PubMed] [Google Scholar]
- 419.McCarthy C, et al. Macrophage PPAR gamma Co-activator-1 alpha participates in repressing foam cell formation and atherosclerosis in response to conjugated linoleic acid. EMBO Mol. Med. 2013;5:1443–1457. doi: 10.1002/emmm.201302587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 420.Zhang X, et al. The m(6)A methyltransferase METTL3 modifies PGC-1alpha mRNA promoting mitochondrial dysfunction and oxLDL-induced inflammation in monocytes. J. Biol. Chem. 2021;297:101058. doi: 10.1016/j.jbc.2021.101058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 421.Minamino T, et al. Vascular cell senescence: contribution to atherosclerosis. Circ. Res. 2007;100:15–26. doi: 10.1161/01.RES.0000256837.40544.4a. [DOI] [PubMed] [Google Scholar]
- 422.Wang J, et al. Vascular Smooth Muscle Cell Senescence Promotes Atherosclerosis and Features of Plaque Vulnerability. Circulation. 2015;132:1909–1919. doi: 10.1161/CIRCULATIONAHA.115.016457. [DOI] [PubMed] [Google Scholar]
- 423.Salazar G, et al. SQSTM1/p62 and PPARGC1A/PGC-1alpha at the interface of autophagy and vascular senescence. Autophagy. 2020;16:1092–1110. doi: 10.1080/15548627.2019.1659612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 424.Erkkinen, M. G. et al. Clinical Neurology and Epidemiology of the Major Neurodegenerative Diseases. Cold Spring Harb. Perspect. Biol. 10, (2018). [DOI] [PMC free article] [PubMed]
- 425.Tritos NA, et al. Characterization of the peroxisome proliferator activated receptor coactivator 1 alpha (PGC 1alpha) expression in the murine brain. Brain Res. 2003;961:255–260. doi: 10.1016/S0006-8993(02)03961-6. [DOI] [PubMed] [Google Scholar]
- 426.Qian K, et al. Cholinergic Neuron Targeting Nanosystem Delivering Hybrid Peptide for Combinatorial Mitochondrial Therapy in Alzheimer’s Disease. ACS Nano. 2022;16:11455–11472. doi: 10.1021/acsnano.2c05795. [DOI] [PubMed] [Google Scholar]
- 427.McMeekin LJ, et al. A Role for PGC-1alpha in Transcription and Excitability of Neocortical and Hippocampal Excitatory Neurons. Neuroscience. 2020;435:73–94. doi: 10.1016/j.neuroscience.2020.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 428.Ye Q, et al. Overexpression of PGC-1alpha Influences Mitochondrial Signal Transduction of Dopaminergic Neurons. Mol. Neurobiol. 2016;53:3756–3770. doi: 10.1007/s12035-015-9299-7. [DOI] [PubMed] [Google Scholar]
- 429.Ciron C, et al. Sustained expression of PGC-1alpha in the rat nigrostriatal system selectively impairs dopaminergic function. Hum. Mol. Genet. 2012;21:1861–1876. doi: 10.1093/hmg/ddr618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 430.Jiang, H. et al. Adult Conditional Knockout of PGC-1alpha Leads to Loss of Dopamine Neurons. eNeuro. 3, (2016). [DOI] [PMC free article] [PubMed]
- 431.Lucas EK, et al. Parvalbumin deficiency and GABAergic dysfunction in mice lacking PGC-1alpha. J. Neurosci. 2010;30:7227–7235. doi: 10.1523/JNEUROSCI.0698-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 432.Vanaveski T, et al. PGC-1alpha Signaling Increases GABA(A) Receptor Subunit alpha2 Expression, GABAergic Neurotransmission and Anxiety-Like Behavior in Mice. Front. Mol. Neurosci. 2021;14:588230. doi: 10.3389/fnmol.2021.588230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 433.Wang J, et al. Adult conditional knockout of PGC-1alpha in GABAergic neurons causes exaggerated startle reactivity, impaired short-term habituation and hyperactivity. Brain Res. Bull. 2020;157:128–139. doi: 10.1016/j.brainresbull.2020.02.005. [DOI] [PubMed] [Google Scholar]
- 434.Dabrowska A, et al. Erratum: PGC-1alpha controls mitochondrial biogenesis and dynamics in lead-induced neurotoxicity. Aging (Albany N. Y). 2015;7:1023. doi: 10.18632/aging.100837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 435.Cheng A, et al. Involvement of PGC-1alpha in the formation and maintenance of neuronal dendritic spines. Nat. Commun. 2012;3:1250. doi: 10.1038/ncomms2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 436.Yang X, et al. Resveratrol regulates microglia M1/M2 polarization via PGC-1alpha in conditions of neuroinflammatory injury. Brain. Behav. Immun. 2017;64:162–172. doi: 10.1016/j.bbi.2017.03.003. [DOI] [PubMed] [Google Scholar]
- 437.Se Thoe E, et al. A review on advances of treatment modalities for Alzheimer’s disease. Life Sci. 2021;276:119129. doi: 10.1016/j.lfs.2021.119129. [DOI] [PubMed] [Google Scholar]
- 438.Ashrafian H, et al. Review on Alzheimer’s disease: Inhibition of amyloid beta and tau tangle formation. Int. J. Biol. Macromol. 2021;167:382–394. doi: 10.1016/j.ijbiomac.2020.11.192. [DOI] [PubMed] [Google Scholar]
- 439.Katsouri L, et al. PPARgamma-coactivator-1alpha gene transfer reduces neuronal loss and amyloid-beta generation by reducing beta-secretase in an Alzheimer’s disease model. Proc. Natl Acad. Sci. Usa. 2016;113:12292–12297. doi: 10.1073/pnas.1606171113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 440.Wang R, et al. Metabolic stress modulates Alzheimer’s beta-secretase gene transcription via SIRT1-PPARgamma-PGC-1 in neurons. Cell Metab. 2013;17:685–694. doi: 10.1016/j.cmet.2013.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 441.Wang J, et al. PGC-1alpha reduces Amyloid-beta deposition in Alzheimer’s disease: Effect of increased VDR expression. Neurosci. Lett. 2021;744:135598. doi: 10.1016/j.neulet.2020.135598. [DOI] [PubMed] [Google Scholar]
- 442.Dong YT, et al. Silent Mating-Type Information Regulation 2 Homolog 1 Attenuates the Neurotoxicity Associated with Alzheimer Disease via a Mechanism Which May Involve Regulation of Peroxisome Proliferator-Activated Receptor Gamma Coactivator 1-alpha. Am. J. Pathol. 2020;190:1545–1564. doi: 10.1016/j.ajpath.2020.03.015. [DOI] [PubMed] [Google Scholar]
- 443.Song T, et al. Mitochondrial dysfunction, oxidative stress, neuroinflammation, and metabolic alterations in the progression of Alzheimer’s disease: A meta-analysis of in vivo magnetic resonance spectroscopy studies. Ageing Res Rev. 2021;72:101503. doi: 10.1016/j.arr.2021.101503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 444.John A, et al. Synaptic basis of Alzheimer’s disease: Focus on synaptic amyloid beta, P-tau and mitochondria. Ageing Res Rev. 2021;65:101208. doi: 10.1016/j.arr.2020.101208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 445.Sheng B, et al. Impaired mitochondrial biogenesis contributes to mitochondrial dysfunction in Alzheimer’s disease. J. Neurochem. 2012;120:419–429. doi: 10.1111/j.1471-4159.2011.07581.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 446.Singulani MP, et al. Impairment of PGC-1alpha-mediated mitochondrial biogenesis precedes mitochondrial dysfunction and Alzheimer’s pathology in the 3xTg mouse model of Alzheimer’s disease. Exp. Gerontol. 2020;133:110882. doi: 10.1016/j.exger.2020.110882. [DOI] [PubMed] [Google Scholar]
- 447.Zhang Y, et al. PPARgamma coactivator-1alpha (PGC-1alpha) protects neuroblastoma cells against amyloid-beta (Abeta) induced cell death and neuroinflammation via NF-kappaB pathway. BMC Neurosci. 2017;18:69. doi: 10.1186/s12868-017-0387-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 448.Dumont M, et al. PGC-1alpha overexpression exacerbates beta-amyloid and tau deposition in a transgenic mouse model of Alzheimer’s disease. FASEB J. 2014;28:1745–1755. doi: 10.1096/fj.13-236331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 449.Kalia LV, et al. Parkinson’s disease. Lancet. 2015;386:896–912. doi: 10.1016/S0140-6736(14)61393-3. [DOI] [PubMed] [Google Scholar]
- 450.Dionisio PA, et al. Oxidative stress and regulated cell death in Parkinson’s disease. Ageing Res Rev. 2021;67:101263. doi: 10.1016/j.arr.2021.101263. [DOI] [PubMed] [Google Scholar]
- 451.Surmeier DJ. Determinants of dopaminergic neuron loss in Parkinson’s disease. FEBS J. 2018;285:3657–3668. doi: 10.1111/febs.14607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 452.Shin JH, et al. PARIS (ZNF746) repression of PGC-1alpha contributes to neurodegeneration in Parkinson’s disease. Cell. 2011;144:689–702. doi: 10.1016/j.cell.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 453.Stevens DA, et al. Parkin loss leads to PARIS-dependent declines in mitochondrial mass and respiration. Proc. Natl Acad. Sci. Usa. 2015;112:11696–11701. doi: 10.1073/pnas.1500624112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 454.Jo, A. et al. PARIS farnesylation prevents neurodegeneration in models of Parkinson’s disease. Sci. Transl. Med. 13, (2021). [DOI] [PMC free article] [PubMed]
- 455.Pirooznia SK, et al. Deubiquitinase CYLD acts as a negative regulator of dopamine neuron survival in Parkinson’s disease. Sci. Adv. 2022;8:eabh1824. doi: 10.1126/sciadv.abh1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 456.Mudo G, et al. Transgenic expression and activation of PGC-1alpha protect dopaminergic neurons in the MPTP mouse model of Parkinson’s disease. Cell Mol. Life Sci. 2012;69:1153–1165. doi: 10.1007/s00018-011-0850-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 457.Sun Z, et al. Characterization of Age-dependent Behavior Deficits in the PGC-1alpha Knockout Mouse, in Relevance to the Parkinson’s Disease Model. Neuroscience. 2020;440:39–47. doi: 10.1016/j.neuroscience.2020.05.015. [DOI] [PubMed] [Google Scholar]
- 458.Ye Q, et al. Mitochondrial Effects of PGC-1alpha Silencing in MPP(+) Treated Human SH-SY5Y Neuroblastoma Cells. Front. Mol. Neurosci. 2017;10:164. doi: 10.3389/fnmol.2017.00164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 459.Wang Y, et al. Beneficial effects of PGC-1alpha in the substantia nigra of a mouse model of MPTP-induced dopaminergic neurotoxicity. Aging (Albany N. Y). 2019;11:8937–8950. doi: 10.18632/aging.102357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 460.Fan F, et al. Regulation of PGC-1alpha mediated by acetylation and phosphorylation in MPP+ induced cell model of Parkinson’s disease. Aging (Albany N. Y). 2020;12:9461–9474. doi: 10.18632/aging.103219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 461.Chen Y, et al. SIRT1 Protects Dopaminergic Neurons in Parkinson’s Disease Models via PGC-1alpha-Mediated Mitochondrial Biogenesis. Neurotox. Res. 2021;39:1393–1404. doi: 10.1007/s12640-021-00392-4. [DOI] [PubMed] [Google Scholar]
- 462.Guan X, et al. PGC-1alpha-siRNA suppresses inflammation in substantia nigra of PD mice by inhibiting microglia. Int. J. Neurosci. 2023;133:269–277. doi: 10.1080/00207454.2021.1910257. [DOI] [PubMed] [Google Scholar]
- 463.Clark J, et al. Pgc-1alpha overexpression downregulates Pitx3 and increases susceptibility to MPTP toxicity associated with decreased Bdnf. PLoS One. 2012;7:e48925. doi: 10.1371/journal.pone.0048925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 464.Surmeier DJ, et al. Selective neuronal vulnerability in Parkinson disease. Nat. Rev. Neurosci. 2017;18:101–113. doi: 10.1038/nrn.2016.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 465.Ciron C, et al. PGC-1alpha activity in nigral dopamine neurons determines vulnerability to alpha-synuclein. Acta Neuropathol. Commun. 2015;3:16. doi: 10.1186/s40478-015-0200-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 466.Eschbach J, et al. Mutual exacerbation of peroxisome proliferator-activated receptor gamma coactivator 1alpha deregulation and alpha-synuclein oligomerization. Ann. Neurol. 2015;77:15–32. doi: 10.1002/ana.24294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 467.Ebrahim AS, et al. Reduced expression of peroxisome-proliferator activated receptor gamma coactivator-1alpha enhances alpha-synuclein oligomerization and down regulates AKT/GSK3beta signaling pathway in human neuronal cells that inducibly express alpha-synuclein. Neurosci. Lett. 2010;473:120–125. doi: 10.1016/j.neulet.2010.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 468.O’Donnell KC, et al. Axon degeneration and PGC-1alpha-mediated protection in a zebrafish model of alpha-synuclein toxicity. Dis. Model Mech. 2014;7:571–582. doi: 10.1242/dmm.013185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 469.Tabrizi SJ, et al. Potential disease-modifying therapies for Huntington’s disease: lessons learned and future opportunities. Lancet Neurol. 2022;21:645–658. doi: 10.1016/S1474-4422(22)00121-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 470.Weydt P, et al. Thermoregulatory and metabolic defects in Huntington’s disease transgenic mice implicate PGC-1alpha in Huntington’s disease neurodegeneration. Cell Metab. 2006;4:349–362. doi: 10.1016/j.cmet.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 471.Taherzadeh-Fard E, et al. PGC-1alpha as modifier of onset age in Huntington disease. Mol. Neurodegener. 2009;4:10. doi: 10.1186/1750-1326-4-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 472.Taherzadeh-Fard E, et al. PGC-1alpha downstream transcription factors NRF-1 and TFAM are genetic modifiers of Huntington disease. Mol. Neurodegener. 2011;6:32. doi: 10.1186/1750-1326-6-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 473.Chaturvedi RK, et al. Transducer of regulated CREB-binding proteins (TORCs) transcription and function is impaired in Huntington’s disease. Hum. Mol. Genet. 2012;21:3474–3488. doi: 10.1093/hmg/dds178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 474.Tsunemi T, et al. PGC-1alpha rescues Huntington’s disease proteotoxicity by preventing oxidative stress and promoting TFEB function. Sci. Transl. Med. 2012;4:142ra197. doi: 10.1126/scitranslmed.3003799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 475.La Spada AR. PPARGC1A/PGC-1alpha, TFEB and enhanced proteostasis in Huntington disease: defining regulatory linkages between energy production and protein-organelle quality control. Autophagy. 2012;8:1845–1847. doi: 10.4161/auto.21862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 476.Cui L, et al. Transcriptional repression of PGC-1alpha by mutant huntingtin leads to mitochondrial dysfunction and neurodegeneration. Cell. 2006;127:59–69. doi: 10.1016/j.cell.2006.09.015. [DOI] [PubMed] [Google Scholar]
- 477.Di Cristo F, et al. Meldonium improves Huntington’s disease mitochondrial dysfunction by restoring peroxisome proliferator-activated receptor gamma coactivator 1alpha expression. J. Cell. Physiol. 2019;234:9233–9246. doi: 10.1002/jcp.27602. [DOI] [PubMed] [Google Scholar]
- 478.Chandra A, et al. Enhanced mitochondrial biogenesis ameliorates disease phenotype in a full-length mouse model of Huntington’s disease. Hum. Mol. Genet. 2016;25:2269–2282. doi: 10.1093/hmg/ddw095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 479.Xiang Z, et al. Peroxisome-proliferator-activated receptor gamma coactivator 1 alpha contributes to dysmyelination in experimental models of Huntington’s disease. J. Neurosci. 2011;31:9544–9553. doi: 10.1523/JNEUROSCI.1291-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 480.Hardiman O, et al. Amyotrophic lateral sclerosis. Nat. Rev. Dis. Prim. 2017;3:17071. doi: 10.1038/nrdp.2017.71. [DOI] [PubMed] [Google Scholar]
- 481.Thau N, et al. Decreased mRNA expression of PGC-1alpha and PGC-1alpha-regulated factors in the SOD1G93A ALS mouse model and in human sporadic ALS. J. Neuropathol. Exp. Neurol. 2012;71:1064–1074. doi: 10.1097/NEN.0b013e318275df4b. [DOI] [PubMed] [Google Scholar]
- 482.Bayer H, et al. ALS-causing mutations differentially affect PGC-1alpha expression and function in the brain vs. peripheral tissues. Neurobiol. Dis. 2017;97:36–45. doi: 10.1016/j.nbd.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 483.Ladd AC, et al. Mitochondrial oxidative phosphorylation transcriptome alterations in human amyotrophic lateral sclerosis spinal cord and blood. Neuromolecular Med. 2014;16:714–726. doi: 10.1007/s12017-014-8321-y. [DOI] [PubMed] [Google Scholar]
- 484.Liang H, et al. PGC-1alpha protects neurons and alters disease progression in an amyotrophic lateral sclerosis mouse model. Muscle Nerve. 2011;44:947–956. doi: 10.1002/mus.22217. [DOI] [PubMed] [Google Scholar]
- 485.Da Cruz S, et al. Elevated PGC-1alpha activity sustains mitochondrial biogenesis and muscle function without extending survival in a mouse model of inherited ALS. Cell Metab. 2012;15:778–786. doi: 10.1016/j.cmet.2012.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 486.Zhao W, et al. Peroxisome proliferator activator receptor gamma coactivator-1alpha (PGC-1alpha) improves motor performance and survival in a mouse model of amyotrophic lateral sclerosis. Mol. Neurodegener. 2011;6:51. doi: 10.1186/1750-1326-6-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 487.Bhargava P, et al. Mitochondrial energetics in the kidney. Nat. Rev. Nephrol. 2017;13:629–646. doi: 10.1038/nrneph.2017.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 488.Tang C, et al. Mitochondrial quality control in kidney injury and repair. Nat. Rev. Nephrol. 2021;17:299–318. doi: 10.1038/s41581-020-00369-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 489.Chambers, J. M. et al. ppargc1a controls nephron segmentation during zebrafish embryonic kidney ontogeny. Elife. 7, (2018). [DOI] [PMC free article] [PubMed]
- 490.Levey AS, et al. Acute Kidney Injury. Ann. Intern Med. 2017;167:ITC66–ITC80. doi: 10.7326/AITC201711070. [DOI] [PubMed] [Google Scholar]
- 491.Tran MT, et al. PGC1alpha drives NAD biosynthesis linking oxidative metabolism to renal protection. Nature. 2016;531:528–532. doi: 10.1038/nature17184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 492.Matejovic M, et al. Molecular differences in susceptibility of the kidney to sepsis-induced kidney injury. BMC Nephrol. 2017;18:183. doi: 10.1186/s12882-017-0602-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 493.Smith JA, et al. Suppression of mitochondrial biogenesis through toll-like receptor 4-dependent mitogen-activated protein kinase kinase/extracellular signal-regulated kinase signaling in endotoxin-induced acute kidney injury. J. Pharm. Exp. Ther. 2015;352:346–357. doi: 10.1124/jpet.114.221085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 494.Thapa K, et al. Targeting ferroptosis in ischemia/reperfusion renal injury. Naunyn Schmiedebergs Arch. Pharmacol. 2022;395:1331–1341. doi: 10.1007/s00210-022-02277-5. [DOI] [PubMed] [Google Scholar]
- 495.Rasbach KA, et al. PGC-1alpha over-expression promotes recovery from mitochondrial dysfunction and cell injury. Biochem Biophys. Res Commun. 2007;355:734–739. doi: 10.1016/j.bbrc.2007.02.023. [DOI] [PubMed] [Google Scholar]
- 496.Funk JA, et al. Accelerated recovery of renal mitochondrial and tubule homeostasis with SIRT1/PGC-1alpha activation following ischemia-reperfusion injury. Toxicol. Appl Pharmacol. 2013;273:345–354. doi: 10.1016/j.taap.2013.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 497.Khader A, et al. Sirtuin 1 activation stimulates mitochondrial biogenesis and attenuates renal injury after ischemia-reperfusion. Transplantation. 2014;98:148–156. doi: 10.1097/TP.0000000000000194. [DOI] [PubMed] [Google Scholar]
- 498.Lempiainen J, et al. Caloric restriction ameliorates kidney ischaemia/reperfusion injury through PGC-1alpha-eNOS pathway and enhanced autophagy. Acta Physiol. (Oxf.). 2013;208:410–421. doi: 10.1111/apha.12120. [DOI] [PubMed] [Google Scholar]
- 499.Wang D, et al. FOXO1 inhibition prevents renal ischemia-reperfusion injury via cAMP-response element binding protein/PPAR-gamma coactivator-1alpha-mediated mitochondrial biogenesis. Br. J. Pharmacol. 2020;177:432–448. doi: 10.1111/bph.14878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 500.Ye P, et al. BMAL1 regulates mitochondrial homeostasis in renal ischaemia-reperfusion injury by mediating the SIRT1/PGC-1alpha axis. J. Cell Mol. Med. 2022;26:1994–2009. doi: 10.1111/jcmm.17223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 501.Peerapanyasut W, et al. Bisphenol A aggravates renal ischemia-reperfusion injury by disrupting mitochondrial homeostasis and N-acetylcysteine mitigates the injurious outcomes. IUBMB Life. 2020;72:758–770. doi: 10.1002/iub.2175. [DOI] [PubMed] [Google Scholar]
- 502.Song YC, et al. Dexmedetomidine Exerts Renal Protective Effect by Regulating the PGC-1alpha/STAT1/IRF-1 Axis. Nephron. 2021;145:528–539. doi: 10.1159/000514532. [DOI] [PubMed] [Google Scholar]
- 503.Barati, A. et al. Eplerenone reduces renal ischaemia/reperfusion injury by modulating Klotho, NF-kappaB and SIRT1/SIRT3/PGC-1alpha signalling pathways. J Pharm Pharmacol. 10.1093/jpp/rgac054 (2022). [DOI] [PubMed]
- 504.Hou J, et al. Treprostinil alleviates hepatic mitochondrial injury during rat renal ischemia-reperfusion injury. Biomed. Pharmacother. 2021;143:112172. doi: 10.1016/j.biopha.2021.112172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 505.Bellomo R, et al. Acute kidney injury in sepsis. Intensive Care Med. 2017;43:816–828. doi: 10.1007/s00134-017-4755-7. [DOI] [PubMed] [Google Scholar]
- 506.van der Slikke EC, et al. Sepsis is associated with mitochondrial DNA damage and a reduced mitochondrial mass in the kidney of patients with sepsis-AKI. Crit. Care. 2021;25:36. doi: 10.1186/s13054-020-03424-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 507.Ruiz-Andres O, et al. The inflammatory cytokine TWEAK decreases PGC-1alpha expression and mitochondrial function in acute kidney injury. Kidney Int. 2016;89:399–410. doi: 10.1038/ki.2015.332. [DOI] [PubMed] [Google Scholar]
- 508.Fontecha-Barriuso M, et al. PGC-1alpha deficiency causes spontaneous kidney inflammation and increases the severity of nephrotoxic AKI. J. Pathol. 2019;249:65–78. doi: 10.1002/path.5282. [DOI] [PubMed] [Google Scholar]
- 509.Yuan L, et al. PGC-1alpha alleviates mitochondrial dysfunction via TFEB-mediated autophagy in cisplatin-induced acute kidney injury. Aging (Albany NY). 2021;13:8421–8439. doi: 10.18632/aging.202653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 510.Li J, et al. Aldehyde dehydrogenase 2 alleviates mitochondrial dysfunction by promoting PGC-1alpha-mediated biogenesis in acute kidney injury. Cell Death Dis. 2023;14:45. doi: 10.1038/s41419-023-05557-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 511.Tong D, et al. Aspirin alleviates cisplatin-induced acute kidney injury through the AMPK-PGC-1alpha signaling pathway. Chem. Biol. Interact. 2023;380:110536. doi: 10.1016/j.cbi.2023.110536. [DOI] [PubMed] [Google Scholar]
- 512.Elkhoely, A. Liraglutide ameliorates gentamicin-induced acute kidney injury in rats via PGC-1alpha- mediated mitochondrial biogenesis: Involvement of PKA/CREB and Notch/Hes-1 signaling pathways. Int Immunopharmacol. 114, 109578 (2023). [DOI] [PubMed]
- 513.Yuan Q, et al. Signaling pathways of chronic kidney diseases, implications for therapeutics. Signal Transduct. Target Ther. 2022;7:182. doi: 10.1038/s41392-022-01036-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 514.Glassock RJ, et al. The global burden of chronic kidney disease: estimates, variability and pitfalls. Nat. Rev. Nephrol. 2017;13:104–114. doi: 10.1038/nrneph.2016.163. [DOI] [PubMed] [Google Scholar]
- 515.Alicic RZ, et al. Diabetic Kidney Disease: Challenges, Progress, and Possibilities. Clin. J. Am. Soc. Nephrol. 2017;12:2032–2045. doi: 10.2215/CJN.11491116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 516.Sharma K, et al. Metabolomics reveals signature of mitochondrial dysfunction in diabetic kidney disease. J. Am. Soc. Nephrol. 2013;24:1901–1912. doi: 10.1681/ASN.2013020126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 517.Qin X, et al. Berberine protects against diabetic kidney disease via promoting PGC-1alpha-regulated mitochondrial energy homeostasis. Br. J. Pharmacol. 2020;177:3646–3661. doi: 10.1111/bph.14935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 518.Wu L, et al. Activation of FoxO1/ PGC-1alpha prevents mitochondrial dysfunction and ameliorates mesangial cell injury in diabetic rats. Mol. Cell Endocrinol. 2015;413:1–12. doi: 10.1016/j.mce.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 519.Guo K, et al. Protective role of PGC-1alpha in diabetic nephropathy is associated with the inhibition of ROS through mitochondrial dynamic remodeling. PLoS One. 2015;10:e0125176. doi: 10.1371/journal.pone.0125176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 520.Hickson LJ, et al. A systematic review and meta-analysis of cell-based interventions in experimental diabetic kidney disease. Stem Cells Transl. Med. 2021;10:1304–1319. doi: 10.1002/sctm.19-0419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 521.Yuan Y, et al. Mitochondrial transfer from mesenchymal stem cells to macrophages restricts inflammation and alleviates kidney injury in diabetic nephropathy mice via PGC-1alpha activation. Stem Cells. 2021;39:913–928. doi: 10.1002/stem.3375. [DOI] [PubMed] [Google Scholar]
- 522.Han, X. et al. Placental Mesenchymal Stem Cells Alleviate Podocyte Injury in Diabetic Kidney Disease by Modulating Mitophagy via the SIRT1-PGC-1alpha-TFAM Pathway. Int J Mol Sci. 24, (2023). [DOI] [PMC free article] [PubMed]
- 523.Kim MY, et al. Resveratrol prevents renal lipotoxicity and inhibits mesangial cell glucotoxicity in a manner dependent on the AMPK-SIRT1-PGC1alpha axis in db/db mice. Diabetologia. 2013;56:204–217. doi: 10.1007/s00125-012-2747-2. [DOI] [PubMed] [Google Scholar]
- 524.Huang Q, et al. Formononetin Attenuates Renal Tubular Injury and Mitochondrial Damage in Diabetic Nephropathy Partly via Regulating Sirt1/PGC-1alpha Pathway. Front Pharmacol. 2022;13:901234. doi: 10.3389/fphar.2022.901234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 525.Wongmekiat, O. et al. Protective Effects of Purple Rice Husk against Diabetic Nephropathy by Modulating PGC-1alpha/SIRT3/SOD2 Signaling and Maintaining Mitochondrial Redox Equilibrium in Rats. Biomolecules. 11, (2021). [DOI] [PMC free article] [PubMed]
- 526.Zhang L, et al. PGC-1alpha ameliorates kidney fibrosis in mice with diabetic kidney disease through an antioxidative mechanism. Mol. Med Rep. 2018;17:4490–4498. doi: 10.3892/mmr.2018.8433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 527.Hou S, et al. Glycyrrhizic Acid Prevents Diabetic Nephropathy by Activating AMPK/SIRT1/PGC-1alpha Signaling in db/db Mice. J. Diabetes Res. 2017;2017:2865912. doi: 10.1155/2017/2865912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 528.Wang JL, et al. Antifibrotic role of PGC-1alpha-siRNA against TGF-beta1-induced renal interstitial fibrosis. Exp. Cell Res. 2018;370:160–167. doi: 10.1016/j.yexcr.2018.06.016. [DOI] [PubMed] [Google Scholar]
- 529.Huang R, et al. Kidney fibrosis: from mechanisms to therapeutic medicines. Signal Transduct. Target Ther. 2023;8:129. doi: 10.1038/s41392-023-01379-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 530.Yang T, et al. YY1 inactivated transcription co-regulator PGC-1alpha to promote mitochondrial dysfunction of early diabetic nephropathy-associated tubulointerstitial fibrosis. Cell Biol. Toxicol. 2023;39:391–413. doi: 10.1007/s10565-022-09711-7. [DOI] [PubMed] [Google Scholar]
- 531.Liu L, et al. Twist1 downregulation of PGC-1alpha decreases fatty acid oxidation in tubular epithelial cells, leading to kidney fibrosis. Theranostics. 2022;12:3758–3775. doi: 10.7150/thno.71722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 532.Zhu, P. et al. ZLN005 Alleviates In Vivo and In Vitro Renal Fibrosis via PGC-1alpha-Mediated Mitochondrial Homeostasis. Pharmaceuticals (Basel). 15, (2022). [DOI] [PMC free article] [PubMed]
- 533.Wang M, et al. Shen Shuai II Recipe attenuates renal fibrosis in chronic kidney disease by improving hypoxia-induced the imbalance of mitochondrial dynamics via PGC-1alpha activation. Phytomedicine. 2022;98:153947. doi: 10.1016/j.phymed.2022.153947. [DOI] [PubMed] [Google Scholar]
- 534.Li, S. Y. et al. Increasing the level of peroxisome proliferator-activated receptor gamma coactivator-1alpha in podocytes results in collapsing glomerulopathy. JCI Insight. 2, (2017). [DOI] [PMC free article] [PubMed]
- 535.Sanchez-de-Diego C, et al. Glucose Restriction Promotes Osteocyte Specification by Activating a PGC-1alpha-Dependent Transcriptional Program. iScience. 2019;15:79–94. doi: 10.1016/j.isci.2019.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 536.Ma JD, et al. Activation of the Peroxisome Proliferator-Activated Receptor gamma Coactivator 1beta/NFATc1 Pathway in Circulating Osteoclast Precursors Associated With Bone Destruction in Rheumatoid Arthritis. Arthritis Rheumatol. 2019;71:1252–1264. doi: 10.1002/art.40868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 537.Ono T, et al. Simultaneous augmentation of muscle and bone by locomomimetism through calcium-PGC-1alpha signaling. Bone Res. 2022;10:52. doi: 10.1038/s41413-022-00225-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 538.Glyn-Jones S, et al. Osteoarthritis. Lancet. 2015;386:376–387. doi: 10.1016/S0140-6736(14)60802-3. [DOI] [PubMed] [Google Scholar]
- 539.Zhao X, et al. Peroxisome proliferator-activated receptor gamma coactivator 1alpha and FoxO3A mediate chondroprotection by AMP-activated protein kinase. Arthritis Rheumatol. 2014;66:3073–3082. doi: 10.1002/art.38791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 540.Wang Y, et al. Mitochondrial biogenesis is impaired in osteoarthritis chondrocytes but reversible via peroxisome proliferator-activated receptor gamma coactivator 1alpha. Arthritis Rheumatol. 2015;67:2141–2153. doi: 10.1002/art.39182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 541.Yang Q, et al. Advanced Glycation End Products Induced Mitochondrial Dysfunction of Chondrocytes through Repression of AMPKalpha-SIRT1-PGC-1alpha Pathway. Pharmacology. 2022;107:298–307. doi: 10.1159/000521720. [DOI] [PubMed] [Google Scholar]
- 542.Feng Z, et al. PPAR-gamma Activation Alleviates Osteoarthritis through Both the Nrf2/NLRP3 and PGC-1alpha/Deltapsi (m) Pathways by Inhibiting Pyroptosis. PPAR Res. 2023;2023:2523536. doi: 10.1155/2023/2523536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 543.Sun J, et al. Sestrin2 overexpression attenuates osteoarthritis pain via induction of AMPK/PGC-1alpha-mediated mitochondrial biogenesis and suppression of neuroinflammation. Brain Behav. Immun. 2022;102:53–70. doi: 10.1016/j.bbi.2022.02.015. [DOI] [PubMed] [Google Scholar]
- 544.Du K, et al. Ferulic acid suppresses interleukin-1beta-induced degeneration of chondrocytes isolated from patients with osteoarthritis through the SIRT1/AMPK/PGC-1alpha signaling pathway. Immun. Inflamm. Dis. 2021;9:710–720. doi: 10.1002/iid3.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 545.Yu M, et al. BMSCs-derived Mitochondria Improve Osteoarthritis by Ameliorating Mitochondrial Dysfunction and Promoting Mitochondrial Biogenesis in Chondrocytes. Stem Cell Rev. Rep. 2022;18:3092–3111. doi: 10.1007/s12015-022-10436-7. [DOI] [PubMed] [Google Scholar]
- 546.Xue S, et al. Cartilage-targeting peptide-modified dual-drug delivery nanoplatform with NIR laser response for osteoarthritis therapy. Bioact. Mater. 2021;6:2372–2389. doi: 10.1016/j.bioactmat.2021.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 547.Duan D, et al. Duchenne muscular dystrophy. Nat. Rev. Dis. Prim. 2021;7:13. doi: 10.1038/s41572-021-00248-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 548.Scholte HR, et al. Muscle mitochondria from patients with Duchenne muscular dystrophy have a normal beta oxidation, but an impaired oxidative phosphorylation. Neurology. 1985;35:1396–1397. doi: 10.1212/WNL.35.9.1396. [DOI] [PubMed] [Google Scholar]
- 549.Kuznetsov AV, et al. Impaired mitochondrial oxidative phosphorylation in skeletal muscle of the dystrophin-deficient mdx mouse. Mol. Cell Biochem. 1998;183:87–96. doi: 10.1023/A:1006868130002. [DOI] [PubMed] [Google Scholar]
- 550.Sampaolesi M, et al. Duchenne cardiomyopathy: targeting ROS and NOX4 as a promising therapeutic strategy. Expert Opin. Ther. Targets. 2023;27:91–95. doi: 10.1080/14728222.2023.2187287. [DOI] [PubMed] [Google Scholar]
- 551.Hughes MC, et al. Early myopathy in Duchenne muscular dystrophy is associated with elevated mitochondrial H(2) O(2) emission during impaired oxidative phosphorylation. J. Cachexia Sarcopenia Muscle. 2019;10:643–661. doi: 10.1002/jcsm.12405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 552.Dubinin MV, et al. Duchenne muscular dystrophy is associated with the inhibition of calcium uniport in mitochondria and an increased sensitivity of the organelles to the calcium-induced permeability transition. Biochim Biophys. Acta Mol. Basis Dis. 2020;1866:165674. doi: 10.1016/j.bbadis.2020.165674. [DOI] [PubMed] [Google Scholar]
- 553.Handschin C, et al. PGC-1alpha regulates the neuromuscular junction program and ameliorates Duchenne muscular dystrophy. Genes Dev. 2007;21:770–783. doi: 10.1101/gad.1525107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 554.Hollinger K, et al. Rescue of dystrophic skeletal muscle by PGC-1alpha involves restored expression of dystrophin-associated protein complex components and satellite cell signaling. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013;305:R13–R23. doi: 10.1152/ajpregu.00221.2012. [DOI] [PubMed] [Google Scholar]
- 555.Hollinger K, et al. PGC-1alpha gene transfer improves muscle function in dystrophic muscle following prolonged disease progress. Exp. Physiol. 2015;100:1145–1158. doi: 10.1113/EP085339. [DOI] [PubMed] [Google Scholar]
- 556.Spaulding HR, et al. PGC-1alpha overexpression increases transcription factor EB nuclear localization and lysosome abundance in dystrophin-deficient skeletal muscle. Physiol. Rep. 2020;8:e14383. doi: 10.14814/phy2.14383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 557.Papadopoulou, S. K. Sarcopenia: A Contemporary Health Problem among Older Adult Populations. Nutrients. 12, (2020). [DOI] [PMC free article] [PubMed]
- 558.Lo JH, et al. Sarcopenia: Current treatments and new regenerative therapeutic approaches. J. Orthop. Transl. 2020;23:38–52. doi: 10.1016/j.jot.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 559.Liu HW, et al. Dysregulations of mitochondrial quality control and autophagic flux at an early age lead to progression of sarcopenia in SAMP8 mice. Biogerontology. 2020;21:367–380. doi: 10.1007/s10522-020-09867-x. [DOI] [PubMed] [Google Scholar]
- 560.Yeo D, et al. Intensified mitophagy in skeletal muscle with aging is downregulated by PGC-1alpha overexpression in vivo. Free Radic. Biol. Med. 2019;130:361–368. doi: 10.1016/j.freeradbiomed.2018.10.456. [DOI] [PubMed] [Google Scholar]
- 561.Guo, M. et al. AAV-Mediated nuclear localized PGC1alpha4 delivery in muscle ameliorates sarcopenia and aging-associated metabolic dysfunctions. Aging Cell. 10.1111/acel.13961e13961 (2023). [DOI] [PMC free article] [PubMed]
- 562.Migliavacca E, et al. Mitochondrial oxidative capacity and NAD(+) biosynthesis are reduced in human sarcopenia across ethnicities. Nat. Commun. 2019;10:5808. doi: 10.1038/s41467-019-13694-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 563.Gilleron J, et al. Endosomal trafficking in metabolic homeostasis and diseases. Nat. Rev. Endocrinol. 2023;19:28–45. doi: 10.1038/s41574-022-00737-9. [DOI] [PubMed] [Google Scholar]
- 564.Amorim JA, et al. Mitochondrial and metabolic dysfunction in ageing and age-related diseases. Nat. Rev. Endocrinol. 2022;18:243–258. doi: 10.1038/s41574-021-00626-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 565.Sattar N, et al. Improving prevention strategies for cardiometabolic disease. Nat. Med. 2020;26:320–325. doi: 10.1038/s41591-020-0786-7. [DOI] [PubMed] [Google Scholar]
- 566.Gerich JE. The genetic basis of type 2 diabetes mellitus: impaired insulin secretion versus impaired insulin sensitivity. Endocr. Rev. 1998;19:491–503. doi: 10.1210/edrv.19.4.0338. [DOI] [PubMed] [Google Scholar]
- 567.Mootha VK, et al. PGC-1alpha-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat. Genet. 2003;34:267–273. doi: 10.1038/ng1180. [DOI] [PubMed] [Google Scholar]
- 568.Hernandez-Alvarez MI, et al. Subjects with early-onset type 2 diabetes show defective activation of the skeletal muscle PGC-1alpha/Mitofusin-2 regulatory pathway in response to physical activity. Diabetes Care. 2010;33:645–651. doi: 10.2337/dc09-1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 569.Choo HJ, et al. Mitochondria are impaired in the adipocytes of type 2 diabetic mice. Diabetologia. 2006;49:784–791. doi: 10.1007/s00125-006-0170-2. [DOI] [PubMed] [Google Scholar]
- 570.Hammarstedt A, et al. Reduced expression of PGC-1 and insulin-signaling molecules in adipose tissue is associated with insulin resistance. Biochem Biophys. Res Commun. 2003;301:578–582. doi: 10.1016/S0006-291X(03)00014-7. [DOI] [PubMed] [Google Scholar]
- 571.Patti ME, et al. Coordinated reduction of genes of oxidative metabolism in humans with insulin resistance and diabetes: Potential role of PGC1 and NRF1. Proc. Natl Acad. Sci. Usa. 2003;100:8466–8471. doi: 10.1073/pnas.1032913100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 572.Rong JX, et al. Adipose mitochondrial biogenesis is suppressed in db/db and high-fat diet-fed mice and improved by rosiglitazone. Diabetes. 2007;56:1751–1760. doi: 10.2337/db06-1135. [DOI] [PubMed] [Google Scholar]
- 573.Liang H, et al. Whole body overexpression of PGC-1alpha has opposite effects on hepatic and muscle insulin sensitivity. Am. J. Physiol. Endocrinol. Metab. 2009;296:E945–E954. doi: 10.1152/ajpendo.90292.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 574.Wu H, et al. Skeletal muscle inflammation and insulin resistance in obesity. J. Clin. Invest. 2017;127:43–54. doi: 10.1172/JCI88880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 575.Sylow L, et al. The many actions of insulin in skeletal muscle, the paramount tissue determining glycemia. Cell Metab. 2021;33:758–780. doi: 10.1016/j.cmet.2021.03.020. [DOI] [PubMed] [Google Scholar]
- 576.Sun C, et al. PCAF improves glucose homeostasis by suppressing the gluconeogenic activity of PGC-1alpha. Cell Rep. 2014;9:2250–2262. doi: 10.1016/j.celrep.2014.11.029. [DOI] [PubMed] [Google Scholar]
- 577.Sharabi K, et al. Selective Chemical Inhibition of PGC-1alpha Gluconeogenic Activity Ameliorates Type 2 Diabetes. Cell. 2017;169:148–160.e115. doi: 10.1016/j.cell.2017.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 578.Yoon JC, et al. Suppression of beta cell energy metabolism and insulin release by PGC-1alpha. Dev. Cell. 2003;5:73–83. doi: 10.1016/S1534-5807(03)00170-9. [DOI] [PubMed] [Google Scholar]
- 579.Valtat B, et al. Fetal PGC-1alpha overexpression programs adult pancreatic beta-cell dysfunction. Diabetes. 2013;62:1206–1216. doi: 10.2337/db12-0314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 580.Feldman EL, et al. Diabetic neuropathy. Nat. Rev. Dis. Prim. 2019;5:41. doi: 10.1038/s41572-019-0092-1. [DOI] [PubMed] [Google Scholar]
- 581.Choi J, et al. PGC-1alpha regulation of mitochondrial degeneration in experimental diabetic neuropathy. Neurobiol. Dis. 2014;64:118–130. doi: 10.1016/j.nbd.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 582.Sawada N, et al. Endothelial PGC-1alpha mediates vascular dysfunction in diabetes. Cell Metab. 2014;19:246–258. doi: 10.1016/j.cmet.2013.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 583.Diniz, A. et al. Type 2 Diabetes Induces a Pro-Oxidative Environment in Rat Epididymis by Disrupting SIRT1/PGC-1alpha/SIRT3 Pathway. Int J Mol Sci. 23, (2022). [DOI] [PMC free article] [PubMed]
- 584.Loos RJF, et al. The genetics of obesity: from discovery to biology. Nat. Rev. Genet. 2022;23:120–133. doi: 10.1038/s41576-021-00414-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 585.Wen X, et al. Signaling pathways in obesity: mechanisms and therapeutic interventions. Signal Transduct. Target Ther. 2022;7:298. doi: 10.1038/s41392-022-01149-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 586.Cypess AM. Reassessing Human Adipose Tissue. N. Engl. J. Med. 2022;386:768–779. doi: 10.1056/NEJMra2032804. [DOI] [PubMed] [Google Scholar]
- 587.Semple RK, et al. Expression of the thermogenic nuclear hormone receptor coactivator PGC-1alpha is reduced in the adipose tissue of morbidly obese subjects. Int J. Obes. Relat. Metab. Disord. 2004;28:176–179. doi: 10.1038/sj.ijo.0802482. [DOI] [PubMed] [Google Scholar]
- 588.Heinonen S, et al. Impaired Mitochondrial Biogenesis in Adipose Tissue in Acquired Obesity. Diabetes. 2015;64:3135–3145. doi: 10.2337/db14-1937. [DOI] [PubMed] [Google Scholar]
- 589.Heinonen S, et al. Mitochondria-related transcriptional signature is downregulated in adipocytes in obesity: a study of young healthy MZ twins. Diabetologia. 2017;60:169–181. doi: 10.1007/s00125-016-4121-2. [DOI] [PubMed] [Google Scholar]
- 590.Shen, S. H. et al. Adipocyte-Specific Expression of PGC1alpha Promotes Adipocyte Browning and Alleviates Obesity-Induced Metabolic Dysfunction in an HO-1-Dependent Fashion. Antioxidants (Basel). 11, (2022). [DOI] [PMC free article] [PubMed]
- 591.Huang, J. et al. Foxj3 regulates thermogenesis of brown and beige fat via induction of PGC-1alpha. Diabetes. 10.2337/db23-0454 (2023). [DOI] [PubMed]
- 592.Son MJ, et al. GATA3 induces the upregulation of UCP-1 by directly binding to PGC-1alpha during adipose tissue browning. Metabolism. 2020;109:154280. doi: 10.1016/j.metabol.2020.154280. [DOI] [PubMed] [Google Scholar]
- 593.Evans, T. D. et al. TFEB drives PGC-1alpha expression in adipocytes to protect against diet-induced metabolic dysfunction. Sci Signal. 12, (2019). [DOI] [PMC free article] [PubMed]
- 594.Ding M, et al. CLCF1 signaling restrains thermogenesis and disrupts metabolic homeostasis by inhibiting mitochondrial biogenesis in brown adipocytes. Proc. Natl Acad. Sci. Usa. 2023;120:e2305717120. doi: 10.1073/pnas.2305717120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 595.Wang Q, et al. IL-27 signalling promotes adipocyte thermogenesis and energy expenditure. Nature. 2021;600:314–318. doi: 10.1038/s41586-021-04127-5. [DOI] [PubMed] [Google Scholar]
- 596.Funda, J. et al. Adipose tissue-specific ablation of PGC-1beta impairs thermogenesis in brown fat. Dis Model Mech. 15, (2022). [DOI] [PMC free article] [PubMed]
- 597.Xu X, et al. Targeted therapeutics and novel signaling pathways in non-alcohol-associated fatty liver/steatohepatitis (NAFL/NASH) Signal Transduct. Target Ther. 2022;7:287. doi: 10.1038/s41392-022-01119-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 598.Dusabimana, T. et al. P2Y2R Deficiency Ameliorates Hepatic Steatosis by Reducing Lipogenesis and Enhancing Fatty Acid beta-Oxidation through AMPK and PGC-1alpha Induction in High-Fat Diet-Fed Mice. Int J Mol Sci. 22, (2021). [DOI] [PMC free article] [PubMed]
- 599.Wu Q, et al. Protein arginine methylation: from enigmatic functions to therapeutic targeting. Nat. Rev. Drug Discov. 2021;20:509–530. doi: 10.1038/s41573-021-00159-8. [DOI] [PubMed] [Google Scholar]
- 600.Park MJ, et al. Thioredoxin-interacting protein mediates hepatic lipogenesis and inflammation via PRMT1 and PGC-1alpha regulation in vitro and in vivo. J. Hepatol. 2014;61:1151–1157. doi: 10.1016/j.jhep.2014.06.032. [DOI] [PubMed] [Google Scholar]
- 601.Xu L, et al. Hepatic PRMT1 ameliorates diet-induced hepatic steatosis via induction of PGC1alpha. Theranostics. 2022;12:2502–2518. doi: 10.7150/thno.63824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 602.Yu C, et al. The role of FOXA family transcription factors in glucolipid metabolism and NAFLD. Front Endocrinol. (Lausanne). 2023;14:1081500. doi: 10.3389/fendo.2023.1081500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 603.Wolfrum C, et al. Coactivation of Foxa2 through Pgc-1beta promotes liver fatty acid oxidation and triglyceride/VLDL secretion. Cell Metab. 2006;3:99–110. doi: 10.1016/j.cmet.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 604.Xia M, et al. Retinol binding protein 4 stimulates hepatic sterol regulatory element-binding protein 1 and increases lipogenesis through the peroxisome proliferator-activated receptor-gamma coactivator 1beta-dependent pathway. Hepatology. 2013;58:564–575. doi: 10.1002/hep.26227. [DOI] [PubMed] [Google Scholar]
- 605.Huang X, et al. Immunohistochemical Analysis of PGC-1alpha and ERRalpha Expression Reveals Their Clinical Significance in Human Ovarian Cancer. Onco Targets Ther. 2020;13:13055–13062. doi: 10.2147/OTT.S288332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 606.Chen L, et al. PGC-1alpha and ERRalpha in patients with endometrial cancer: a translational study for predicting myometrial invasion. Aging (Albany NY). 2020;12:16963–16980. doi: 10.18632/aging.103611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 607.Cai FF, et al. Prognostic value of plasma levels of HIF-1a and PGC-1a in breast cancer. Oncotarget. 2016;7:77793–77806. doi: 10.18632/oncotarget.12796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 608.Cruz-Bermudez A, et al. PGC-1alpha levels correlate with survival in patients with stage III NSCLC and may define a new biomarker to metabolism-targeted therapy. Sci. Rep. 2017;7:16661. doi: 10.1038/s41598-017-17009-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 609.Xiang Y, et al. SR18292 exerts potent antitumor effects in multiple myeloma via inhibition of oxidative phosphorylation. Life Sci. 2020;256:117971. doi: 10.1016/j.lfs.2020.117971. [DOI] [PubMed] [Google Scholar]
- 610.Do MT, et al. Metformin induces microRNA-34a to downregulate the Sirt1/Pgc-1alpha/Nrf2 pathway, leading to increased susceptibility of wild-type p53 cancer cells to oxidative stress and therapeutic agents. Free Radic. Biol. Med. 2014;74:21–34. doi: 10.1016/j.freeradbiomed.2014.06.010. [DOI] [PubMed] [Google Scholar]
- 611.Wang, C. W. et al. Aqueous Extract of Paris polyphylla (AEPP) Inhibits Ovarian Cancer via Suppression of Peroxisome Proliferator-Activated Receptor-Gamma Coactivator (PGC)-1alpha. Molecules. 21, (2016). [DOI] [PMC free article] [PubMed]
- 612.Yu M, et al. Isoliquiritigenin inhibits gastric cancer growth through suppressing GLUT4 mediated glucose uptake and inducing PDHK1/PGC-1alpha mediated energy metabolic collapse. Phytomedicine. 2023;121:155045. doi: 10.1016/j.phymed.2023.155045. [DOI] [PubMed] [Google Scholar]
- 613.Xu YH, et al. Bouchardatine suppresses rectal cancer in mice by disrupting its metabolic pathways via activating the SIRT1-PGC-1alpha-UCP2 axis. Eur. J. Pharmacol. 2019;854:328–337. doi: 10.1016/j.ejphar.2019.04.029. [DOI] [PubMed] [Google Scholar]
- 614.Ghoneim HE, et al. Cell-Intrinsic Barriers of T Cell-Based Immunotherapy. Trends Mol. Med. 2016;22:1000–1011. doi: 10.1016/j.molmed.2016.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 615.Lisci M, et al. Arming a killer: mitochondrial regulation of CD8(+) T cell cytotoxicity. Trends Cell Biol. 2023;33:138–147. doi: 10.1016/j.tcb.2022.05.007. [DOI] [PubMed] [Google Scholar]
- 616.Chamoto K, et al. Mitochondrial activation chemicals synergize with surface receptor PD-1 blockade for T cell-dependent antitumor activity. Proc. Natl Acad. Sci. Usa. 2017;114:E761–E770. doi: 10.1073/pnas.1620433114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 617.Chowdhury PS, et al. PPAR-Induced Fatty Acid Oxidation in T Cells Increases the Number of Tumor-Reactive CD8(+) T Cells and Facilitates Anti-PD-1 Therapy. Cancer Immunol. Res. 2018;6:1375–1387. doi: 10.1158/2326-6066.CIR-18-0095. [DOI] [PubMed] [Google Scholar]
- 618.Wan H, et al. PGC-1alpha activator-induced fatty acid oxidation in tumor-infiltrating CTLs enhances effects of PD-1 blockade therapy in lung cancer. Tumori. 2020;106:55–63. doi: 10.1177/0300891619868287. [DOI] [PubMed] [Google Scholar]
- 619.Dumauthioz N, et al. Enforced PGC-1alpha expression promotes CD8 T cell fitness, memory formation and antitumor immunity. Cell Mol. Immunol. 2021;18:1761–1771. doi: 10.1038/s41423-020-0365-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 620.Zhong X, et al. Ncoa2 Promotes CD8+ T cell-Mediated Antitumor Immunity by Stimulating T-cell Activation via Upregulation of PGC-1alpha Critical for Mitochondrial Function. Cancer Immunol. Res. 2023;11:1414–1431. doi: 10.1158/2326-6066.CIR-23-0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 621.Zhang H, et al. Ketogenesis-generated beta-hydroxybutyrate is an epigenetic regulator of CD8(+) T-cell memory development. Nat. Cell Biol. 2020;22:18–25. doi: 10.1038/s41556-019-0440-0. [DOI] [PubMed] [Google Scholar]
- 622.Malinee M, et al. Targeted epigenetic induction of mitochondrial biogenesis enhances antitumor immunity in mouse model. Cell Chem. Biol. 2022;29:463–475 e466. doi: 10.1016/j.chembiol.2021.08.001. [DOI] [PubMed] [Google Scholar]
- 623.Lontos, K. et al. Metabolic reprogramming via an engineered PGC-1alpha improves human chimeric antigen receptor T-cell therapy against solid tumors. J Immunother Cancer. 11, (2023). [DOI] [PMC free article] [PubMed]
- 624.Weydt P, et al. A single nucleotide polymorphism in the coding region of PGC-1alpha is a male-specific modifier of Huntington disease age-at-onset in a large European cohort. BMC Neurol. 2014;14:1. doi: 10.1186/1471-2377-14-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 625.Weydt P, et al. The gene coding for PGC-1alpha modifies age at onset in Huntington’s Disease. Mol. Neurodegener. 2009;4:3. doi: 10.1186/1750-1326-4-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 626.Che HV, et al. Localization of sequence variations in PGC-1alpha influence their modifying effect in Huntington disease. Mol. Neurodegener. 2011;6:1. doi: 10.1186/1750-1326-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 627.Pasquinelli A, et al. Gly482Ser PGC-1alpha Gene Polymorphism and Exercise-Related Oxidative Stress in Amyotrophic Lateral Sclerosis Patients. Front Cell Neurosci. 2016;10:102. doi: 10.3389/fncel.2016.00102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 628.Kunej T, et al. A Gly482Ser polymorphism of the peroxisome proliferator-activated receptor-gamma coactivator-1 (PGC-1) gene is associated with type 2 diabetes in Caucasians. Folia Biol. (Praha). 2004;50:157–158. [PubMed] [Google Scholar]
- 629.Andrulionyte L, et al. Common polymorphisms of the PPAR-gamma2 (Pro12Ala) and PGC-1alpha (Gly482Ser) genes are associated with the conversion from impaired glucose tolerance to type 2 diabetes in the STOP-NIDDM trial. Diabetologia. 2004;47:2176–2184. doi: 10.1007/s00125-004-1577-2. [DOI] [PubMed] [Google Scholar]
- 630.Sharma R, et al. Association of PGC-1alpha gene with type 2 diabetes in three unrelated endogamous groups of North-West India (Punjab): a case-control and meta-analysis study. Mol. Genet Genomics. 2018;293:317–329. doi: 10.1007/s00438-017-1385-2. [DOI] [PubMed] [Google Scholar]
- 631.Jemaa Z, et al. The Gly482Ser polymorphism of the peroxisome proliferator-activated receptor-gamma coactivator-1alpha (PGC-1alpha) is associated with type 2 diabetes in Tunisian population. Diabetes Metab. Syndr. 2015;9:316–319. doi: 10.1016/j.dsx.2013.10.011. [DOI] [PubMed] [Google Scholar]
- 632.Wu HH, et al. Association and interaction analysis of PPARGC1A and serum uric acid on type 2 diabetes mellitus in Chinese Han population. Diabetol. Metab. Syndr. 2014;6:107. doi: 10.1186/1758-5996-6-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 633.Shokouhi S, et al. Association between PGC-1alpha gene polymorphisms and type 2 diabetes risk: a case-control study of an Iranian population. Can. J. Diabetes. 2015;39:65–72. doi: 10.1016/j.jcjd.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 634.Weng SW, et al. Gly482Ser polymorphism in the peroxisome proliferator-activated receptor gamma coactivator-1alpha gene is associated with oxidative stress and abdominal obesity. Metabolism. 2010;59:581–586. doi: 10.1016/j.metabol.2009.08.021. [DOI] [PubMed] [Google Scholar]
- 635.Gayathri SB, et al. Association of the PPARGC1A gene polymorphism with diabetic nephropathy in an Asian Indian population (CURES-41) Metab. Syndr. Relat. Disord. 2010;8:119–126. doi: 10.1089/met.2009.0040. [DOI] [PubMed] [Google Scholar]
- 636.Wang S, et al. Polymorphisms of the peroxisome proliferator-activated receptor-gamma coactivator-1alpha gene are associated with hypertrophic cardiomyopathy and not with hypertension hypertrophy. Clin. Chem. Lab Med. 2007;45:962–967. doi: 10.1515/CCLM.2007.189. [DOI] [PubMed] [Google Scholar]
- 637.Bhat A, et al. PGC-1alpha Thr394Thr and Gly482Ser variants are significantly associated with T2DM in two North Indian populations: a replicate case-control study. Hum. Genet. 2007;121:609–614. doi: 10.1007/s00439-007-0352-0. [DOI] [PubMed] [Google Scholar]
- 638.Sun L, et al. The Gly482Ser variant of the PPARGC1 gene is associated with Type 2 diabetes mellitus in northern Chinese, especially men. Diabet. Med. 2006;23:1085–1092. doi: 10.1111/j.1464-5491.2006.01949.x. [DOI] [PubMed] [Google Scholar]
- 639.Lin YC, et al. A common variant in the peroxisome proliferator-activated receptor-gamma coactivator-1alpha gene is associated with nonalcoholic fatty liver disease in obese children. Am. J. Clin. Nutr. 2013;97:326–331. doi: 10.3945/ajcn.112.046417. [DOI] [PubMed] [Google Scholar]
- 640.Ambye L, et al. Studies of the Gly482Ser polymorphism of the peroxisome proliferator-activated receptor gamma coactivator 1alpha (PGC-1alpha) gene in Danish subjects with the metabolic syndrome. Diabetes Res Clin. Pract. 2005;67:175–179. doi: 10.1016/j.diabres.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 641.Huang M, et al. Engineered allele substitution at PPARGC1A rs8192678 alters human white adipocyte differentiation, lipogenesis, and PGC-1alpha content and turnover. Diabetologia. 2023;66:1289–1305. doi: 10.1007/s00125-023-05915-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 642.Hong YA, et al. Resveratrol Ameliorates Contrast Induced Nephropathy Through the Activation of SIRT1-PGC-1alpha-Foxo1 Signaling in Mice. Kidney Blood Press Res. 2017;42:641–653. doi: 10.1159/000481804. [DOI] [PubMed] [Google Scholar]
- 643.Yang, K. et al. Resveratrol Attenuates Hyperoxia Lung Injury in Neonatal Rats by Activating SIRT1/PGC-1alpha Signaling Pathway. Am J Perinatol. 10.1055/a-1787-3396 (2022). [DOI] [PubMed]
- 644.Fang WJ, et al. Resveratrol improves diabetic cardiomyopathy by preventing asymmetric dimethylarginine-caused peroxisome proliferator-activated receptor-gamma coactivator-1alpha acetylation. Eur. J. Pharmacol. 2022;936:175342. doi: 10.1016/j.ejphar.2022.175342. [DOI] [PubMed] [Google Scholar]
- 645.Fang WJ, et al. Resveratrol alleviates diabetic cardiomyopathy in rats by improving mitochondrial function through PGC-1alpha deacetylation. Acta Pharm. Sin. 2018;39:59–73. doi: 10.1038/aps.2017.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 646.Zheng, M. et al. Resveratrol Reestablishes Mitochondrial Quality Control in Myocardial Ischemia/Reperfusion Injury through Sirt1/Sirt3-Mfn2-Parkin-PGC-1alpha Pathway. Molecules. 27, (2022). [DOI] [PMC free article] [PubMed]
- 647.Zhang T, et al. Resveratrol Reduces Oxidative Stress and Apoptosis in Podocytes via Sir2-Related Enzymes, Sirtuins1 (SIRT1)/Peroxisome Proliferator-Activated Receptor gamma Co-Activator 1alpha (PGC-1alpha) Axis. Med Sci. Monit. 2019;25:1220–1231. doi: 10.12659/MSM.911714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 648.Li J, et al. Resveratrol Prevents ROS-Induced Apoptosis in High Glucose-Treated Retinal Capillary Endothelial Cells via the Activation of AMPK/Sirt1/PGC-1alpha Pathway. Oxid. Med Cell Longev. 2017;2017:7584691. doi: 10.1155/2017/7584691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 649.Nishigaki A, et al. Resveratrol protects mitochondrial quantity by activating SIRT1/PGC-1alpha expression during ovarian hypoxia. Reprod. Med Biol. 2020;19:189–197. doi: 10.1002/rmb2.12323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 650.Zhai X, et al. Curcumin regulates peroxisome proliferator-activated receptor-gamma coactivator-1alpha expression by AMPK pathway in hepatic stellate cells in vitro. Eur. J. Pharmacol. 2015;746:56–62. doi: 10.1016/j.ejphar.2014.10.055. [DOI] [PubMed] [Google Scholar]
- 651.Li Y, et al. Curcumin attenuates isoniazid-induced hepatotoxicity by upregulating the SIRT1/PGC-1alpha/NRF1 pathway. J. Appl Toxicol. 2022;42:1192–1204. doi: 10.1002/jat.4288. [DOI] [PubMed] [Google Scholar]
- 652.Wu Y, et al. Curcumin Relieves Chronic Unpredictable Mild Stress-Induced Depression-Like Behavior through the PGC-1alpha/FNDC5/BDNF Pathway. Behav. Neurol. 2021;2021:2630445. doi: 10.1155/2021/2630445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 653.Wang X, et al. Hypoxic preconditioning combined with curcumin promotes cell survival and mitochondrial quality of bone marrow mesenchymal stem cells, and accelerates cutaneous wound healing via PGC-1alpha/SIRT3/HIF-1alpha signaling. Free Radic. Biol. Med. 2020;159:164–176. doi: 10.1016/j.freeradbiomed.2020.07.023. [DOI] [PubMed] [Google Scholar]
- 654.Barinda AJ, et al. Curcumin Prevents Epithelial-to Mesenchymal Transition-Mediated Ovarian Cancer Progression through NRF2/ETBR/ET-1 Axis and Preserves Mitochondria Biogenesis in Kidney after Cisplatin Administration. Adv. Pharm. Bull. 2022;12:128–141. doi: 10.34172/apb.2022.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 655.Xu J, et al. Berberine Acts on C/EBPbeta/lncRNA Gas5/miR-18a-5p Loop to Decrease the Mitochondrial ROS Generation in HK-2 Cells. Front Endocrinol. (Lausanne). 2021;12:675834. doi: 10.3389/fendo.2021.675834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 656.Yerra VG, et al. Adenosine monophosphate-activated protein kinase modulation by berberine attenuates mitochondrial deficits and redox imbalance in experimental diabetic neuropathy. Neuropharmacology. 2018;131:256–270. doi: 10.1016/j.neuropharm.2017.12.029. [DOI] [PubMed] [Google Scholar]
- 657.Shi, Z. et al. Berberine Protects against NEFA-Induced Impairment of Mitochondrial Respiratory Chain Function and Insulin Signaling in Bovine Hepatocytes. Int J Mol Sci. 19, (2018). [DOI] [PMC free article] [PubMed]
- 658.Yao S, et al. Berberine attenuates the abnormal ectopic lipid deposition in skeletal muscle. Free Radic. Biol. Med. 2020;159:66–75. doi: 10.1016/j.freeradbiomed.2020.07.028. [DOI] [PubMed] [Google Scholar]
- 659.Yu Y, et al. Berberine Improves Cognitive Deficiency and Muscular Dysfunction via Activation of the AMPK/SIRT1/PGC-1a Pathway in Skeletal Muscle from Naturally Aging Rats. J. Nutr. Health Aging. 2018;22:710–717. doi: 10.1007/s12603-018-1015-7. [DOI] [PubMed] [Google Scholar]
- 660.Yang M, et al. Berberine Ameliorates Cognitive Disorder via GSK3beta/PGC-1alpha Signaling in APP/PS1 Mice. J. Nutr. Sci. Vitaminol. (Tokyo). 2022;68:228–235. doi: 10.3177/jnsv.68.228. [DOI] [PubMed] [Google Scholar]
- 661.Li X, et al. Neuroprotection by quercetin via mitochondrial function adaptation in traumatic brain injury: PGC-1alpha pathway as a potential mechanism. J. Cell Mol. Med. 2018;22:883–891. doi: 10.1111/jcmm.13313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 662.Liu P, et al. Quercetin ameliorates hypobaric hypoxia-induced memory impairment through mitochondrial and neuron function adaptation via the PGC-1alpha pathway. Restor. Neurol. Neurosci. 2015;33:143–157. doi: 10.3233/RNN-140446. [DOI] [PubMed] [Google Scholar]
- 663.Ho, C. L. et al. Quercetin Increases Mitochondrial Biogenesis and Reduces Free Radicals in Neuronal SH-SY5Y Cells. Nutrients. 14, (2022). [DOI] [PMC free article] [PubMed]
- 664.Hsu, M. Y. et al. Quercetin Alleviates the Accumulation of Superoxide in Sodium Iodate-Induced Retinal Autophagy by Regulating Mitochondrial Reactive Oxygen Species Homeostasis through Enhanced Deacetyl-SOD2 via the Nrf2-PGC-1alpha-Sirt1 Pathway. Antioxidants (Basel). 10, (2021). [DOI] [PMC free article] [PubMed]
- 665.Tang J, et al. Quercetin improve ischemia/reperfusion-induced cardiomyocyte apoptosis in vitro and in vivo study via SIRT1/PGC-1alpha signaling. J. Cell Biochem. 2019;120:9747–9757. doi: 10.1002/jcb.28255. [DOI] [PubMed] [Google Scholar]
- 666.Sharma DR, et al. Quercetin protects against aluminium induced oxidative stress and promotes mitochondrial biogenesis via activation of the PGC-1alpha signaling pathway. Neurotoxicology. 2015;51:116–137. doi: 10.1016/j.neuro.2015.10.002. [DOI] [PubMed] [Google Scholar]
- 667.Peng, J. et al. Quercetin Reprograms Immunometabolism of Macrophages via the SIRT1/PGC-1alpha Signaling Pathway to Ameliorate Lipopolysaccharide-Induced Oxidative Damage. Int J Mol Sci. 24, (2023). [DOI] [PMC free article] [PubMed]
- 668.Zhao X, et al. Quercetin Protects Ethanol-Induced Hepatocyte Pyroptosis via Scavenging Mitochondrial ROS and Promoting PGC-1alpha-Regulated Mitochondrial Homeostasis in L02 Cells. Oxid. Med Cell Longev. 2022;2022:4591134. doi: 10.1155/2022/4591134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 669.Comakli S, et al. Beneficial effects of quercetin on vincristine-induced liver injury in rats: Modulating the levels of Nrf2/HO-1, NF-kB/STAT3, and SIRT1/PGC-1alpha. J. Biochem Mol. Toxicol. 2023;37:e23326. doi: 10.1002/jbt.23326. [DOI] [PubMed] [Google Scholar]
- 670.Xie, M. et al. Astragaloside IV ameliorates peritoneal fibrosis by promoting PGC-1alpha to reduce apoptosis in vitro and in vivo. J Cell Mol Med. 10.1111/jcmm.17871 (2023). [DOI] [PMC free article] [PubMed]
- 671.Jiang B, et al. Astragaloside IV reverses simvastatin-induced skeletal muscle injury by activating the AMPK-PGC-1alpha signalling pathway. Phytother. Res. 2020;34:1175–1184. doi: 10.1002/ptr.6593. [DOI] [PubMed] [Google Scholar]
- 672.Zhang S, et al. Astragaloside IV protects against isoproterenol-induced cardiac hypertrophy by regulating NF-kappaB/PGC-1alpha signaling mediated energy biosynthesis. PLoS One. 2015;10:e0118759. doi: 10.1371/journal.pone.0118759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 673.Jin X, et al. Baicalin ameliorates CUMS-induced depression-like behaviors through activating AMPK/PGC-1alpha pathway and enhancing NIX-mediated mitophagy in mice. Eur. J. Pharmacol. 2023;938:175435. doi: 10.1016/j.ejphar.2022.175435. [DOI] [PubMed] [Google Scholar]
- 674.Fu X, et al. A New Perspective on Ameliorating Depression-Like Behaviors: Suppressing Neuroinflammation by Upregulating PGC-1alpha. Neurotox. Res. 2021;39:872–885. doi: 10.1007/s12640-020-00292-z. [DOI] [PubMed] [Google Scholar]
- 675.Fang P, et al. Baicalin ameliorates hepatic insulin resistance and gluconeogenic activity through inhibition of p38 MAPK/PGC-1alpha pathway. Phytomedicine. 2019;64:153074. doi: 10.1016/j.phymed.2019.153074. [DOI] [PubMed] [Google Scholar]
- 676.Fujiwara, T. et al. PGC-1alpha-mediated angiogenesis prevents pulmonary hypertension in mice. JCI Insight. 8, (2023). [DOI] [PMC free article] [PubMed]
- 677.Leng Q, et al. Dihydromyricetin ameliorates diet-induced obesity and promotes browning of white adipose tissue by upregulating IRF4/PGC-1alpha. Nutr. Metab. (Lond.). 2022;19:38. doi: 10.1186/s12986-022-00672-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 678.Han H, et al. Dihydromyricetin Protects Against Gentamicin-Induced Ototoxicity via PGC-1alpha/SIRT3 Signaling in vitro. Front Cell Dev. Biol. 2020;8:702. doi: 10.3389/fcell.2020.00702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 679.Huang Y, et al. Dihydromyricetin Attenuates Dexamethasone-Induced Muscle Atrophy by Improving Mitochondrial Function via the PGC-1alpha Pathway. Cell Physiol. Biochem. 2018;49:758–779. doi: 10.1159/000493040. [DOI] [PubMed] [Google Scholar]
- 680.Shi L, et al. Dihydromyricetin improves skeletal muscle insulin sensitivity by inducing autophagy via the AMPK-PGC-1alpha-Sirt3 signaling pathway. Endocrine. 2015;50:378–389. doi: 10.1007/s12020-015-0599-5. [DOI] [PubMed] [Google Scholar]
- 681.Silva J, et al. Dihydromyricetin improves mitochondrial outcomes in the liver of alcohol-fed mice via the AMPK/Sirt-1/PGC-1alpha signaling axis. Alcohol. 2021;91:1–9. doi: 10.1016/j.alcohol.2020.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 682.Wang L, et al. Isoliquiritigenin-mediated miR-23a-3p inhibition activates PGC-1alpha to alleviate alcoholic liver injury. Phytomedicine. 2022;96:153845. doi: 10.1016/j.phymed.2021.153845. [DOI] [PubMed] [Google Scholar]
- 683.Wang L, et al. Activation of PGC-1alpha via isoliquiritigenin-induced downregulation of miR-138-5p alleviates nonalcoholic fatty liver disease. Phytother. Res. 2022;36:899–913. doi: 10.1002/ptr.7334. [DOI] [PubMed] [Google Scholar]
- 684.Luan A, et al. Astragalus polysaccharide attenuates isoproterenol-induced cardiac hypertrophy by regulating TNF-alpha/PGC-1alpha signaling mediated energy biosynthesis. Environ. Toxicol. Pharmacol. 2015;39:1081–1090. doi: 10.1016/j.etap.2015.03.014. [DOI] [PubMed] [Google Scholar]
- 685.Gu C, et al. Astragalus polysaccharides affect insulin resistance by regulating the hepatic SIRT1-PGC-1alpha/PPARalpha-FGF21 signaling pathway in male Sprague Dawley rats undergoing catch-up growth. Mol. Med Rep. 2015;12:6451–6460. doi: 10.3892/mmr.2015.4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 686.Li F, et al. Dexmedetomidine reduces oxidative stress and provides neuroprotection in a model of traumatic brain injury via the PGC-1alpha signaling pathway. Neuropeptides. 2018;72:58–64. doi: 10.1016/j.npep.2018.10.004. [DOI] [PubMed] [Google Scholar]
- 687.Huang J, et al. Dexmedetomidine Protects Against Neurological Dysfunction in a Mouse Intracerebral Hemorrhage Model by Inhibiting Mitochondrial Dysfunction-Derived Oxidative Stress. J. Stroke Cerebrovasc. Dis. 2019;28:1281–1289. doi: 10.1016/j.jstrokecerebrovasdis.2019.01.016. [DOI] [PubMed] [Google Scholar]
- 688.Yu JL, et al. Dexmedetomidine alleviates doxorubicin cardiotoxicity by inhibiting mitochondrial reactive oxygen species generation. Hum. Cell. 2020;33:47–56. doi: 10.1007/s13577-019-00282-0. [DOI] [PubMed] [Google Scholar]
- 689.Shao Q, et al. Dexmedetomidine protects cardiac microvascular endothelial cells from the damage of ogd/r through regulation of the ppardelta-mediated autophagy. Microcirculation. 2021;28:e12675. doi: 10.1111/micc.12675. [DOI] [PubMed] [Google Scholar]
- 690.Chuang, Y. C. et al. Resveratrol Promotes Mitochondrial Biogenesis and Protects against Seizure-Induced Neuronal Cell Damage in the Hippocampus Following Status Epilepticus by Activation of the PGC-1alpha Signaling Pathway. Int J Mol Sci. 20, (2019). [DOI] [PMC free article] [PubMed]
- 691.Lang J, et al. Resveratrol Attenuated Manganese-Induced Learning and Memory Impairments in Mice Through PGC-1Alpha-Mediated Autophagy and Microglial M1/M2 Polarization. Neurochem Res. 2022;47:3414–3427. doi: 10.1007/s11064-022-03695-w. [DOI] [PubMed] [Google Scholar]
- 692.Zhou J, et al. Resveratrol Improves Mitochondrial Biogenesis Function and Activates PGC-1alpha Pathway in a Preclinical Model of Early Brain Injury Following Subarachnoid Hemorrhage. Front Mol. Biosci. 2021;8:620683. doi: 10.3389/fmolb.2021.620683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 693.Feng X, et al. Berberine in Cardiovascular and Metabolic Diseases: From Mechanisms to Therapeutics. Theranostics. 2019;9:1923–1951. doi: 10.7150/thno.30787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 694.Zhi W, et al. Melatonin elicits protective effects on OGD/R‑insulted H9c2 cells by activating PGC‑1alpha/Nrf2 signaling. Int J. Mol. Med. 2020;45:1294–1304. doi: 10.3892/ijmm.2020.4514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 695.Qi X, et al. Melatonin improves mitochondrial biogenesis through the AMPK/PGC1alpha pathway to attenuate ischemia/reperfusion-induced myocardial damage. Aging (Albany NY). 2020;12:7299–7312. doi: 10.18632/aging.103078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 696.Yang Y, et al. Melatonin alleviates angiotensin-II-induced cardiac hypertrophy via activating MICU1 pathway. Aging (Albany NY). 2020;13:493–515. doi: 10.18632/aging.202159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 697.Lee JH, et al. Melatonin-Induced PGC-1alpha Improves Angiogenic Potential of Mesenchymal Stem Cells in Hindlimb Ischemia. Biomol. Ther. (Seoul.). 2020;28:240–249. doi: 10.4062/biomolther.2019.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 698.Luan P, et al. Melatonin relieves 2,2,4,4-tetrabromodiphenyl ether (BDE-47)-induced apoptosis and mitochondrial dysfunction through the AMPK-Sirt1-PGC-1alpha axis in fish kidney cells (CIK) Ecotoxicol. Environ. Saf. 2022;232:113276. doi: 10.1016/j.ecoenv.2022.113276. [DOI] [PubMed] [Google Scholar]
- 699.Yu LM, et al. Melatonin attenuates diabetic cardiomyopathy and reduces myocardial vulnerability to ischemia-reperfusion injury by improving mitochondrial quality control: Role of SIRT6. J. Pineal Res. 2021;70:e12698. doi: 10.1111/jpi.12698. [DOI] [PubMed] [Google Scholar]
- 700.Yu L, et al. Melatonin ameliorates myocardial ischemia/reperfusion injury in type 1 diabetic rats by preserving mitochondrial function: role of AMPK-PGC-1alpha-SIRT3 signaling. Sci. Rep. 2017;7:41337. doi: 10.1038/srep41337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 701.Han B, et al. Dietary melatonin attenuates chromium-induced lung injury via activating the Sirt1/Pgc-1alpha/Nrf2 pathway. Food Funct. 2019;10:5555–5565. doi: 10.1039/C9FO01152H. [DOI] [PubMed] [Google Scholar]
- 702.Niu YJ, et al. Melatonin enhances mitochondrial biogenesis and protects against rotenone-induced mitochondrial deficiency in early porcine embryos. J. Pineal Res. 2020;68:e12627. doi: 10.1111/jpi.12627. [DOI] [PubMed] [Google Scholar]
- 703.Dong W, et al. Melatonin improves mitochondrial function by preventing mitochondrial fission in cadmium-induced rat proximal tubular cell injury via SIRT1-PGC-1alpha pathway activation. Ecotoxicol. Environ. Saf. 2022;242:113879. doi: 10.1016/j.ecoenv.2022.113879. [DOI] [PubMed] [Google Scholar]
- 704.Yao Y, et al. Melatonin attenuates bisphenol A-induced colon injury by dual targeting mitochondrial dynamics and Nrf2 antioxidant system via activation of SIRT1/PGC-1alpha signaling pathway. Free Radic. Biol. Med. 2023;195:13–22. doi: 10.1016/j.freeradbiomed.2022.12.081. [DOI] [PubMed] [Google Scholar]
- 705.Sun D, et al. Metformin improves cardiac function in mice with heart failure after myocardial infarction by regulating mitochondrial energy metabolism. Biochem Biophys. Res Commun. 2017;486:329–335. doi: 10.1016/j.bbrc.2017.03.036. [DOI] [PubMed] [Google Scholar]
- 706.Ashabi G, et al. Activation of AMP-activated protein kinase by metformin protects against global cerebral ischemia in male rats: interference of AMPK/PGC-1alpha pathway. Metab. Brain Dis. 2014;29:47–58. doi: 10.1007/s11011-013-9475-2. [DOI] [PubMed] [Google Scholar]
- 707.Li Q, et al. Metformin-induced autophagy and irisin improves INS-1 cell function and survival in high-glucose environment via AMPK/SIRT1/PGC-1alpha signal pathway. Food Sci. Nutr. 2019;7:1695–1703. doi: 10.1002/fsn3.1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 708.Khan MP, et al. Pathophysiological Mechanism of Bone Loss in Type 2 Diabetes Involves Inverse Regulation of Osteoblast Function by PGC-1alpha and Skeletal Muscle Atrogenes: AdipoR1 as a Potential Target for Reversing Diabetes-Induced Osteopenia. Diabetes. 2015;64:2609–2623. doi: 10.2337/db14-1611. [DOI] [PubMed] [Google Scholar]
- 709.Pilegaard H, et al. Exercise induces transient transcriptional activation of the PGC-1alpha gene in human skeletal muscle. J. Physiol. 2003;546:851–858. doi: 10.1113/jphysiol.2002.034850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 710.Short KR, et al. Impact of aerobic exercise training on age-related changes in insulin sensitivity and muscle oxidative capacity. Diabetes. 2003;52:1888–1896. doi: 10.2337/diabetes.52.8.1888. [DOI] [PubMed] [Google Scholar]
- 711.Terada S, et al. Effects of acute bouts of running and swimming exercise on PGC-1alpha protein expression in rat epitrochlearis and soleus muscle. Am. J. Physiol. Endocrinol. Metab. 2004;286:E208–E216. doi: 10.1152/ajpendo.00051.2003. [DOI] [PubMed] [Google Scholar]
- 712.Taylor EB, et al. Endurance training increases skeletal muscle LKB1 and PGC-1alpha protein abundance: effects of time and intensity. Am. J. Physiol. Endocrinol. Metab. 2005;289:E960–E968. doi: 10.1152/ajpendo.00237.2005. [DOI] [PubMed] [Google Scholar]
- 713.Suwa M, et al. Endurance exercise increases the SIRT1 and peroxisome proliferator-activated receptor gamma coactivator-1alpha protein expressions in rat skeletal muscle. Metabolism. 2008;57:986–998. doi: 10.1016/j.metabol.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 714.Lira VA, et al. PGC-1alpha regulation by exercise training and its influences on muscle function and insulin sensitivity. Am. J. Physiol. Endocrinol. Metab. 2010;299:E145–E161. doi: 10.1152/ajpendo.00755.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 715.Handschin C. Peroxisome proliferator-activated receptor gamma coactivator 1beta (PGC-1beta) improves skeletal muscle mitochondrial function and insulin sensitivity. Diabetologia. 2011;54:1270–1272. doi: 10.1007/s00125-011-2135-3. [DOI] [PubMed] [Google Scholar]
- 716.Mathai AS, et al. Rapid exercise-induced changes in PGC-1alpha mRNA and protein in human skeletal muscle. J. Appl Physiol. (1985). 2008;105:1098–1105. doi: 10.1152/japplphysiol.00847.2007. [DOI] [PubMed] [Google Scholar]
- 717.Neto IVS, et al. Pleiotropic and multi-systemic actions of physical exercise on PGC-1alpha signaling during the aging process. Ageing Res Rev. 2023;87:101935. doi: 10.1016/j.arr.2023.101935. [DOI] [PubMed] [Google Scholar]
- 718.Hofer SJ, et al. The ups and downs of caloric restriction and fasting: from molecular effects to clinical application. EMBO Mol. Med. 2022;14:e14418. doi: 10.15252/emmm.202114418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 719.Nisoli E, et al. Calorie restriction promotes mitochondrial biogenesis by inducing the expression of eNOS. Science. 2005;310:314–317. doi: 10.1126/science.1117728. [DOI] [PubMed] [Google Scholar]
- 720.Baker DJ, et al. No decline in skeletal muscle oxidative capacity with aging in long-term calorically restricted rats: effects are independent of mitochondrial DNA integrity. J. Gerontol. A Biol. Sci. Med Sci. 2006;61:675–684. doi: 10.1093/gerona/61.7.675. [DOI] [PubMed] [Google Scholar]
- 721.Ranhotra HS. Long-term caloric restriction up-regulates PPAR gamma co-activator 1 alpha (PGC-1alpha) expression in mice. Indian J. Biochem Biophys. 2010;47:272–277. [PubMed] [Google Scholar]
- 722.Teng CT, et al. Fasting induces the expression of PGC-1alpha and ERR isoforms in the outer stripe of the outer medulla (OSOM) of the mouse kidney. PLoS One. 2011;6:e26961. doi: 10.1371/journal.pone.0026961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 723.Hempenstall S, et al. Dietary restriction increases skeletal muscle mitochondrial respiration but not mitochondrial content in C57BL/6 mice. Mech. Ageing Dev. 2012;133:37–45. doi: 10.1016/j.mad.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 724.Waldman M, et al. Regulation of diabetic cardiomyopathy by caloric restriction is mediated by intracellular signaling pathways involving ‘SIRT1 and PGC-1alpha’. Cardiovasc Diabetol. 2018;17:111. doi: 10.1186/s12933-018-0754-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 725.Ma, L. et al. Long-term caloric restriction activates the myocardial SIRT1/AMPK/PGC-1alpha pathway in C57BL/6J male mice. Food Nutr Res. 64, (2020). [DOI] [PMC free article] [PubMed]
- 726.Masternak MM, et al. Caloric restriction results in decreased expression of peroxisome proliferator-activated receptor superfamily in muscle of normal and long-lived growth hormone receptor/binding protein knockout mice. J. Gerontol. A Biol. Sci. Med Sci. 2005;60:1238–1245. doi: 10.1093/gerona/60.10.1238. [DOI] [PubMed] [Google Scholar]
- 727.Caro P, et al. Effect of 40% restriction of dietary amino acids (except methionine) on mitochondrial oxidative stress and biogenesis, AIF and SIRT1 in rat liver. Biogerontology. 2009;10:579–592. doi: 10.1007/s10522-008-9200-4. [DOI] [PubMed] [Google Scholar]
- 728.DeSimone JD, et al. Survey of ophthalmic imaging use to assess risk of progression of choroidal nevus to melanoma. Eye (Lond.). 2023;37:953–958. doi: 10.1038/s41433-022-02110-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 729.Finley LW, et al. Skeletal muscle transcriptional coactivator PGC-1alpha mediates mitochondrial, but not metabolic, changes during calorie restriction. Proc. Natl Acad. Sci. Usa. 2012;109:2931–2936. doi: 10.1073/pnas.1115813109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 730.Wong KE, et al. Muscle-Specific Overexpression of PGC-1alpha Does Not Augment Metabolic Improvements in Response to Exercise and Caloric Restriction. Diabetes. 2015;64:1532–1543. doi: 10.2337/db14-0827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 731.Lin J, et al. Hyperlipidemic effects of dietary saturated fats mediated through PGC-1beta coactivation of SREBP. Cell. 2005;120:261–273. doi: 10.1016/j.cell.2004.11.043. [DOI] [PubMed] [Google Scholar]
- 732.Russell AP. PGC-1alpha and exercise: important partners in combating insulin resistance. Curr. Diabetes Rev. 2005;1:175–181. doi: 10.2174/1573399054022811. [DOI] [PubMed] [Google Scholar]
- 733.Choi CS, et al. Paradoxical effects of increased expression of PGC-1alpha on muscle mitochondrial function and insulin-stimulated muscle glucose metabolism. Proc. Natl Acad. Sci. Usa. 2008;105:19926–19931. doi: 10.1073/pnas.0810339105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 734.Achi, I. T. et al. Multi-Target Potential of Berberine as an Antineoplastic and Antimetastatic Agent: A Special Focus on Lung Cancer Treatment. Cells. 11, (2022). [DOI] [PMC free article] [PubMed]
- 735.Parsamanesh N, et al. Resveratrol and endothelial function: A literature review. Pharm. Res. 2021;170:105725. doi: 10.1016/j.phrs.2021.105725. [DOI] [PubMed] [Google Scholar]
- 736.Li H, et al. Curcumin, the golden spice in treating cardiovascular diseases. Biotechnol. Adv. 2020;38:107343. doi: 10.1016/j.biotechadv.2019.01.010. [DOI] [PubMed] [Google Scholar]
- 737.Suzuki Y, et al. ZLN005 improves the survival of polymicrobial sepsis by increasing the bacterial killing via inducing lysosomal acidification and biogenesis in phagocytes. Front Immunol. 2023;14:1089905. doi: 10.3389/fimmu.2023.1089905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 738.Pang X, et al. SIRT3 ameliorates polycystic ovary syndrome through FOXO1/PGC-1alpha signaling pathway. Endocrine. 2023;80:201–211. doi: 10.1007/s12020-022-03262-x. [DOI] [PubMed] [Google Scholar]
- 739.Li Y, et al. Deoxyarbutin attenuates severe acute pancreatitis via the HtrA2/PGC-1alpha pathway. Free Radic. Res. 2022;56:651–665. doi: 10.1080/10715762.2022.2163244. [DOI] [PubMed] [Google Scholar]
- 740.Lawniczak A, et al. Aging and short-term calorie restriction differently affect the cardiac and skeletal muscle expression of genes regulating energy substrate utilization in male rats. Biogerontology. 2022;23:325–340. doi: 10.1007/s10522-022-09965-y. [DOI] [PubMed] [Google Scholar]
- 741.Hao W, et al. Ligand-Modified Gold Nanoparticles as Mitochondrial Modulators: Regulation of Intestinal Barrier and Therapy for Constipation. ACS Nano. 2023;17:13377–13392. doi: 10.1021/acsnano.3c01656. [DOI] [PubMed] [Google Scholar]
- 742.Cai D, et al. Nanoparticle endothelial delivery of PGC-1alpha attenuates hypoxia-induced pulmonary hypertension by attenuating EndoMT-caused vascular wall remodeling. Redox Biol. 2022;58:102524. doi: 10.1016/j.redox.2022.102524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 743.Rahman, M. M. et al. Melatonin Supplement Plus Exercise Effectively Counteracts the Challenges of Isoproterenol-Induced Cardiac Injury in Rats. Biomedicines. 11, (2023). [DOI] [PMC free article] [PubMed]
- 744.Xiao M, et al. Calorie Restriction Combined with High-Intensity Interval Training Promotes Browning of White Adipose Tissue by Activating the PPARgamma/PGC-1alpha/UCP1 Pathway. Alter. Ther. Health Med. 2023;29:134–139. [PubMed] [Google Scholar]
- 745.Northam C, et al. Metabolic regulation by the PGC-1alpha and PGC-1beta coactivators in larval zebrafish (Danio rerio) Comp. Biochem Physiol. A Mol. Integr. Physiol. 2019;234:60–67. doi: 10.1016/j.cbpa.2019.04.011. [DOI] [PubMed] [Google Scholar]
- 746.Kurchaba N, et al. Metabolic consequences of PGC-1alpha dysregulation in adult zebrafish muscle. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2022;323:R319–R330. doi: 10.1152/ajpregu.00188.2021. [DOI] [PubMed] [Google Scholar]