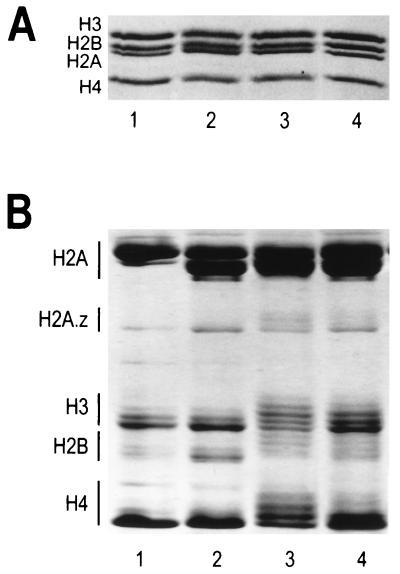
FIG. 1.
Analysis of core histone octamers purified from HeLa cells and chicken erythrocytes. (A) SDS-polyacrylamide gel electrophoresis. Two micrograms of purified histone octamers were electrophoresed on an SDS–18% polyacrylamide gel (37), and bands were visualized by staining with Coomassie blue G-250. Samples were loaded as follows: lane 1, chicken erythrocyte octamers; lane 2, underacetylated octamers isolated from untreated HeLa cells; lane 3, highly acetylated octamers isolated from fraction A of butyrate-treated HeLa cells (see Materials and Methods); lane 4, moderately acetylated octamers isolated from fraction C of butyrate-treated HeLa cells. (B) Resolution of acetylated histone species. Fifteen micrograms of the histone octamers from panel A were electrophoresed on a 6 M acetic acid–6 M urea–0.375% Triton X-100–polyacrylamide gel (12) for 16 h at 5 mA of constant current. The bands were visualized by Coomassie blue G-250 staining. Lanes 1 to 4 correspond to the same histone octamers as in panel A. Histones H2B, H3, and H4 each have four acetylation sites, while H2A has only one acetylation site (53). For each core histone (with the exception of H2A), increasing extents of acetylation lead to progressively slower band migration. In the case of histone H2A, the slower-migrating band corresponds to H2A.1 while the faster-migrating band corresponds to the H2A.2 variant (65).