Figure 1.
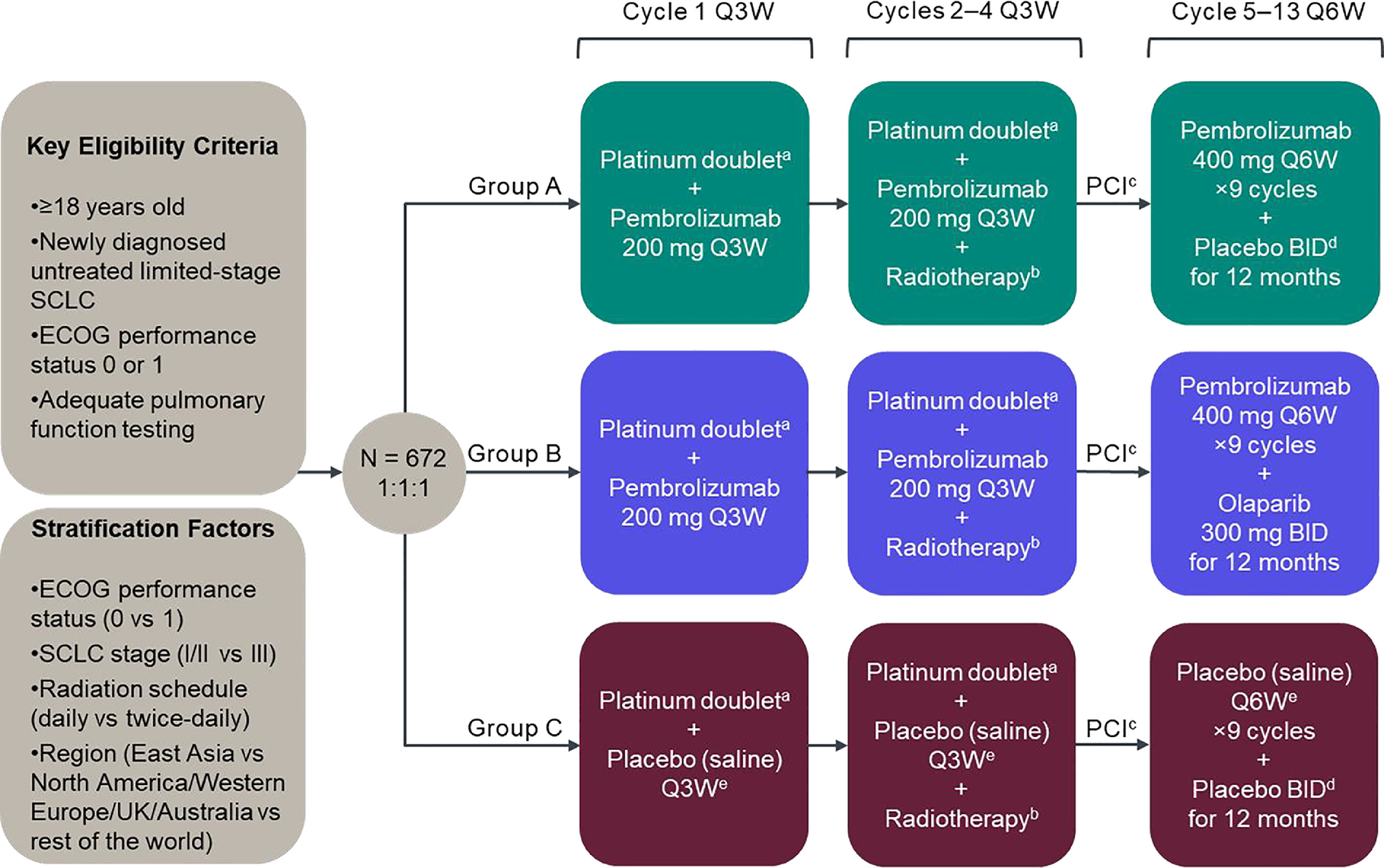
KEYLYNK-013 study design. AUC, area under the curve; BID, twice daily; CCRT, concurrent chemoradiotherapy; ECOG, Eastern Cooperative Oncology Group; PCI, prophylactic cranial irradiation; Q3W, every 3 weeks; Q6W, every 6 weeks; UK, United Kingdom. aPlatinum doublet chemotherapy comprises etoposide 100 mg/m2 on days 1, 2 and 3 of each cycle and investigator’s choice of carboplatin AUC 5 mg/mL/min or cisplatin 75 mg/m2 on day 1 of each cycle. Chemotherapy treatments will be administered intravenously Q3W for 4 cycles. bRadiotherapy is administered twice daily (45 Gy in 30 twice-daily fractions of 1.5 Gy over 3 weeks, 5 days/week) or daily (66 Gy in 33 daily fractions of 2 Gy over 6.5 weeks, 5 days/week), starting concurrently with chemotherapy on day 1 of cycle 2. cPCI occurs after early response assessment (imaging 12 weeks after randomization) and is strongly recommended for participants who achieve complete or partial response following CCRT and is at the investigator’s discretion for participants with stable disease. PCI should be administered no later than 6 weeks after the last dose of cycle 4. The standard dose for PCI to the whole brain is 25 Gy in 10 daily fractions (2.5 Gy/fraction). dOlaparib matching placebo. ePembrolizumab matching placebo.
