Abstract
Background:
Neutrophil-lymphocyte ratio (NLR) has been associated with mortality in several disease sites. We hypothesized that NLR is associated with inferior outcomes in localized non-small cell lung cancer (NSCLC) treated with stereotactic body radiotherapy (SBRT).
Methods:
We evaluated the association of pre-treatment NLR, obtained within 6 months of starting SBRT, with overall survival, as well as primary tumor, regional, and distant recurrence. Multivariate Cox regression was then used to assess pre-treatment NLR as a predictor of mortality. We validated our findings in an independent cohort of patients treated at two other institutions. In a secondary analysis, we also evaluated the association of post-treatment NLR with mortality in the training cohort.
Results:
A total of 156 patients and 166 tumors were included in the training cohort with a median follow-up of 13.4 months. After dichotomization by median, NLR > 3.6 was associated with mortality on univariate (p = 0.010) and multivariate analysis (p = 0.023). In the validation cohort, NLR > 3.6 was similarly associated with mortality on univariate (p = 0.031) and multivariate (p = 0.007) analysis. In a secondary analysis in the training cohort, we found post-treatment NLR was significantly increased compared to pre-treatment NLR (p < 0.001) and associated with mortality on univariate analysis (p = 0.005) and multivariate analysis (p = 0.010).
Conclusions:
Pre-treatment NLR > 3.6 is associated with mortality in patients treated with SBRT. This finding was validated in an independent cohort of patients treated at two other institutions. Additionally, post-treatment NLR was significantly increased from pre-treatment and associated with overall survival.
Keywords: Neutrophil–lymphocyte ratio, Stereotactic body radiation therapy, Non-small cell lung cancer
Non-small cell lung cancer (NSCLC) is a leading cause of cancer-related death worldwide. About 16% of cases are considered early stage at the time of diagnosis [1]. With the recent implementation of the U.S. Preventative Services Task Force (USPSTF) Lung Cancer Screening Guidelines, more cases of NSCLC will likely be detected at an earlier stage [2]. The primary treatment for early-stage NSCLC is surgical resection. However, in patients with unfavorable characteristics such as poor performance status, inadequate pulmonary function, or multiple medical co-morbidities, stereotactic body radiotherapy (SBRT) provides a promising alternative. Although there are no completed randomized trials, many retrospective studies have demonstrated comparable clinical success following SBRT, with local tumor control reported to be above 90% at 2–5 years following treatment [3–4]. Nevertheless, in the group of inoperable patients for whom SBRT is commonly prescribed, overall survival is still poor [3].
There are many well-described tumor-, treatment-, and host-related prognostic biomarkers for NSCLC. Examples of tumor- and treatment-factors include TNM stage [5], histology [6], and biologically effective dose of radiation [7]. Host-related factors such as age [8], Eastern Cooperative Oncology Group (ECOG) performance status, and smoking status [9] provide additional data in forecasting prognosis. In this select population of patients treated with SBRT, patients are generally characterized by early-stage disease burden and unfavorable host characteristics that preclude surgery. Therefore, there is a need to identify additional prognostic markers in this population to optimize risk stratification and guide management decisions.
Neutrophil-lymphocyte ratio (NLR) is an emerging biomarker of interest for several malignancies, and is readily assessed from a serum complete blood count (CBC) with differential. Elevated pre-treatment NLR has been associated with poor outcomes in many cancers, with the highest significance in mesothelioma followed by pancreatic, renal cell carcinoma and colorectal cancer [10]. Multiple studies have also investigated and supported the prognostic utility of NLR in NSCLC treated with surgery or chemotherapy [11–13]. Recent studies have suggested a prognostic role of NLR in the setting of lung SBRT [14–17]. None of these studies, however, demonstrated statistical significance of a clinically meaningful cutoff value of NLR when adjusting for covariates, which is likely a function of the relatively smaller sample size of patients with available CBC data. In addition, none of these studies rigorously validated their findings and statistical cut-point using an independent cohort of patients. Because these limitations undermine the clinical utility of such findings, we sought to evaluate the association of NLR with survival and validate our findings multi-institutionally, while accounting for clinically relevant variables. Finally, we evaluated the changes in NLR after SBRT and investigated the role of post-treatment NLR in prognosticating clinical outcomes, which has not been previously explored.
Materials and methods
Patient selection
This study was an Institutional Review Board (IRB)-approved chart review of patients with localized NSCLC treated at The Ohio State University James Cancer Hospital. Patient data including demographics, staging, pathology, and serum laboratory values were extracted from the electronic medical record. Inclusion criteria consisted of (1) histologically confirmed, localized NSCLC, (2) N0M0 disease, (3) considered medically inoperable or patient refused surgery, (4) treatment with SBRT to a biologically effective dose (BEDGy10) > 100 Gy. We included patients treated with up to 8 fractions due to the institutional use of hypofractionated radiotherapy for central tumors [18]. Additionally, we included a few (n = 6) patients with T3 or T4 tumors due to multifocal tumors attributed to the same primary. We validated our findings in a cohort of patients treated at Massachusetts General Hospital and Rutgers Cancer Institute of New Jersey using the same inclusion criteria, under IRB approval at these institutions. Since routine CBCs were not performed prior to SBRT, we initially analyzed all patients with an available CBC with differential within 6 months prior to starting treatment. To limit the potential variability of the NLR-to-treatment interval, a subset analysis was performed for patients with available NLR within 3 months prior to starting treatment. Similarly, in the analysis of post-treatment NLR, all patients had an available CBC with differential within 6 months after completion of treatment, and a subset analysis was performed of patients within 2 months of starting treatment.
Staging and treatment
Tumors were staged according to the American Joint Committee on Cancer (AJCC) guidelines, 7th edition [19]. Clinical staging included positron emission tomography (PET) and EBUS or CT-guided biopsy and/or mediastinal staging.
For treatment planning, free-breathing and four-dimensional (4D) computed tomography (CT) simulation scans with or without contrast were performed at all institutions. The gross tumor volume (GTV) was contoured on the free-breathing scan or 50% phase of the 4D-CT scan. Both the internal target volumes (ITV) and planning target volumes (PTV) were generated from the 4D scan. A 5-mm expansion from the ITV was typically used to produce the PTV. Standard maximum dose constraints from multi-institutional protocols were respected for organs at risk (OARs) [3–4,20]. Patients received post-treatment imaging with either CT or PET at 2–3 months to evaluate response. There were subsequent follow-up visits every 3–6 months for the first two years and then every 6 months thereafter.
Statistical analysis
Pre-treatment NLR, calculated as the division of absolute neutrophil count (ANC) by the absolute lymphocyte count (ALC), was derived from the most recent CBC with differential within six months prior to starting SBRT. Post-treatment NLR was obtained from a CBC drawn one to six months after treatment. Patients were dichotomized by the median pre-treatment NLR value. Patient characteristics for those patients with NLR above- and below-the-median were compared using Fisher’s exact test and Wilcoxon’s rank sum test for categorical and continuous variables, respectively. The difference between pre- and post-NLR was compared using Wilcoxon’s sign rank test.
The primary outcome was overall survival (OS), calculated from the date of last SBRT treatment to the most recent follow-up or date of death. Secondary outcomes included time to primary tumor, regional, and distant failure. Primary tumor failure was defined as failure at the site of the treated tumor as determined by PET scan, biopsy, and/or consensus of a multidisciplinary tumor board. Regional nodal failure was defined as recurrence in the regional nodes including the mediastinal and hilar basins. Distant failure was defined as recurrence at sites other than the treated lobe and regional nodes.
Log-rank and cox regression were used to evaluate the association between NLR and clinical outcomes. Kaplan–Meier curves were generated for overall survival, primary tumor failure, regional nodal failure, and distant metastasis. Cox regression multivariate analysis was performed including age, gender, T stage, histology, ECOG performance status, Charlson Comorbidity Index, smoking and BEDGy10 as covariates. Comorbidity scores were unavailable for the validation cohort. Hazard ratios (HR) and 95% confidence intervals (CI) were reported. Hypothesis tests were two-sided, with a significance threshold of P < 0.05. Data analysis was performed using SAS (version 9.4, SAS Institute Inc., Cary, NC).
Results
Pre-treatment NLR as a prognostic biomarker
Data from 156 patients and a total of 166 treated tumors treated at The Ohio State University James Cancer Hospital were analyzed with a median post-treatment follow-up time of 13.4 months (Table 1). Eighty-nine patients were male (57%) and 67 were female (43%), with a median age of 72 (range 51–92). The majority of treated tumors were T1 (n = 115, 69.3%) followed by T2 (n = 37, 35.8%), and T3 (n = 6, 3.6%) and T4 (n = 8, 4.8%). The median total dose, dose-per-fraction, number of fractions, and BEDGy10 of treatment were 50 Gy (range 45–60 Gy), 12 Gy (range 7.5–18.7 Gy), 5 fractions (range 3–8), and 105.6 Gy (range 100–160.5 Gy), respectively.
Table 1.
Baseline patient, treatment, and tumor characteristics of the training cohort, stratified by NLR ≤ 3.6 and NLR > 3.6 groups.*
| Variable | All Patients (N = 156) |
NLR ≤ 3.6 (N = 82) |
NLR > 3.6 (N = 74) |
P value† |
|---|---|---|---|---|
| Pretreatment NLR | ||||
| Median (range) | 3.60 (0.2, 41.8) | 2.4 (2.0, 3.0) | 5.4 (4.3, 8.7) | <.0001 |
| Age | ||||
| Median (range) | 72 years (51, 92) | 72 (65.75, 77.25) | 70 (65.75, 77.25) | 0.859 |
| Gender | ||||
| Male | 89 (57%) | 46 (56.1%) | 43 (58.1%) | 0.87 |
| Female | 67 (43%) | 36 (43.9%) | 31 (41.9%) | |
| Charlson Comorbidity Index | ||||
| Median (range) | 6 (3, 13) | 6 (3, 13) | 6 (3, 11) | 0.80 |
| ECOG Performance Status | ||||
| 0 | 30 (19.2%) | 19 (23.1%) | 11 (14.9%) | 0.3 |
| 1 | 76 (48.7%) | 42 (51.2%) | 34 (45.9%) | |
| 2 | 41 (26.3%) | 18 (22 %) | 23 (31.08%) | |
| 3 | 8 (5.1%) | 3 (3.7%) | 5(6.8%) | |
| 4 | 1 (0.6%) | 0 (0%) | 1 (1.3%) | |
| Race | ||||
| White | 129 (82.7%) | 63 (76.8%) | 66 (89.2%) | 0.08 |
| Black | 25 (16.0%) | 17 (20.7%) | 8 (10.8%) | |
| Other | 2 (1.3%) | 2 (2.4%) | 0 (0%) | |
| History of Smoking | ||||
| No | 9 (5.8%) | 4 (4.9%) | 5 (6.8%) | 0.74 |
| Yes | 147 (94.2%) | 78 (95.1%) | 69 (93.2%) | |
| All Tumors (N = 166) |
NLR ≤ 3.6 (N = 86) |
NLR > 3.6 (N = 80) |
p-value† | |
|
| ||||
| Histology | ||||
| Squamous cell carcinoma | 59 (35.5%) | 31 (36.1%) | 28 (35%) | 0.81 |
| Adenocarcinoma | 89 (53.0%) | 44 (51.2%) | 44 (55%) | |
| NSCLC, NOS | 19 (11.5%) | 11 (12.8%) | 8 (10.0%) | |
| T-stage‡ | ||||
| T1a | 66 (39.8%) | 32 (37.2%) | 34 (42.5%) | 0.31 |
| T1b | 49 (29.5%) | 26 (30.2%) | 23 (28.8%) | |
| T2a | 34 (34%) | 22 (25.6%) | 12 (15%) | |
| T2b | 3 (1.8%) | 2 (2.3%) | 1 (1.25%) | |
| T3 | 6 (3.6%) | 2 (2.3%) | 4 (5%) | |
| T4 | 8 (4.8%) | 2 (2.3%) | 6 (7.5%) | |
| GTV (cm3) | ||||
| Median (range) | 7.62 (0.54, 158.02) | 7.25 (0.76, 55.5) | 7.75 (0.54, 158.02) | 0.79 |
| PTV (cm3) | ||||
| Median (range) | 36.0 (3.9, 266.2) | 35.9 (3.9, 166.8) | 36.2 (7.4, 266.2) | 0.57 |
| Total BED (Gy10) | ||||
| Median (range) | 105.6 (100, 160.5) | 112.5 (100, 160.5) | 105.0 (100, 151.2) | 0.07 |
| Max SUV on Pre-Treatment PET¥ | ||||
| Median (range) | 9.5 (1, 28.2) | 9.9 (1, 27.7) | 8.7 (1.6, 28.2) | 0.43 |
| WBC (K/μL) | ||||
| Median (range) | 7.5 (2.5, 141.1) | 7.0 (2.5, 32.6) | 8.66 (3.9, 141.1) | 0.0001 |
| Neutrophil Count (K/μL) | ||||
| Median (range) | 5.04 (1.03, 118.3) | 4.45 (1, 15.8) | 6.68 (2.55, 118.4) | <0.0001 |
| Lymphocyte Count (K/μL) | ||||
| Median (range) | 1.39 (0.28, 27.1) | 1.74 (0.79, 27.1) | 1.11 (0.28, 10.6) | <0.0001 |
Abbreviations. NLR – neutrophil–lymphocyte ratio. ECOG – Eastern Cooperative Group. NSCLC – non-small cell lung cancer, not otherwise specified. GTV – gross tumor volume. PTV – planning target volume. BED – biologic effective dose. SUV – standardized uptake value. PET – Positron Emission Tomography. WBC – white blood count.
Continuous variables were tested by Mann–Whitney U test, discrete variables were tested by Fisher’s Exact tests.
American Joint Committee on Cancer (AJCC) 7th edition.
Total of 149 with available values (neutrophil–lymphocyte ratio > 3.6, n = 75; neutrophil–lymphocyte ratio ≤ 3.6, n = 74.
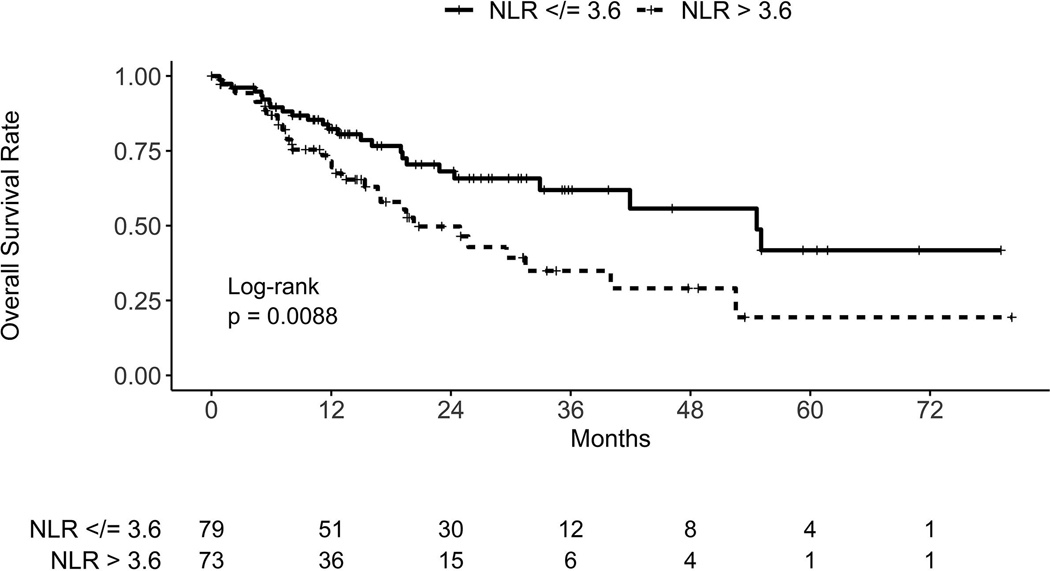
The median overall survival time of the entire cohort was 32.9 months (95% CI 24.3, upper bound not reached). The median NLR of the cohort was 3.6 (range 0.2–41.8), obtained at a median of 1.6 months (range 0.1–5.6) before starting SBRT. The median overall survival time for patients with NLR below/equal to and above the median was 54.6 months (95% CI 32.9, upper bound not reached) and 20.3 months (95% CI 15.4–40), respectively (Fig. 1). On univariate cox regression analysis, we found a statistically significant association of pre-treatment NLR > 3.6 with worse overall survival (HR = 2.00, 95% CI 1.18–3.39, p = 0.010). On Cox multivariate analysis, NLR > 3.6 continued to be statistically and independently associated with inferior overall survival (HR = 1.91, 95% CI 1.09–3.33, p = 0.023) when accounting for age, gender, T-stage, histology, ECOG performance status, Charlson Comorbidity Index, smoking, and BEDGy10 (Table 2). There was no statistically significant association of pre-treatment NLR > 3.6 with primary tumor failure (HR = 1.21, 95% CI 0.42–3.49, p = 0.73), regional nodal failure (HR = 1.30, 95% CI 0.61–2.74, p = 0.50), or distant failure (HR = 1.95, 95% CI 0.81–4.72, p = 0.14) (see Supplementary Figs. 1–3). In a subset analysis of the 131 patients with NLR obtained within 3 months (median of 1.3 months) before starting treatment, NLR > 3.6 continued to be associated with inferior overall survival on univariate (HR = 2.34; 95% CI 1.30–4.18; p = 0.004) and multivariate (HR = 2.14; 95% CI 1.15–3.99; p = 0.016) analysis.
Fig. 1.
Kaplan–Meier curves for overall survival in the training cohort, stratified by neutrophil–lymphocyte ratio (NLR) group.
Table 2.
Cox proportional hazards multivariate analysis of predictors for survival in the training cohort.*
| Variable | HR (95% CI) | p-value |
|---|---|---|
| NLR | ||
| ≤3.6 | 1.0 | – |
| >3.6 | 1.91 (1.09–3.33) | 0.023 |
| Age | 1.02 (0.98–1.06) | 0.26 |
| Gender | ||
| Male | 1.0 | – |
| Female | 0.85 (0.47–1.55) | 0.60 |
| T-stage† | ||
| T1a | 1.0 | – |
| T1b | 0.87 (0.44–1.68) | 0.67 |
| T2a | 0.95 (0.42–2.15) | 0.91 |
| T2b | 1.82 (0.23–14.43) | 0.57 |
| T3 | 1.22 (0.25–5.95) | 0.81 |
| T4 | 0.52 (0.07–4.00) | 0.53 |
| Histology | ||
| SCC | 1.0 | – |
| Adenocarcinoma | 0.91 (0.49–1.69) | 0.77 |
| NSCLC, NOS | 0.94 (0.38–2.34) | 0.89 |
| Total BEDGy10 | 0.99 (0.97–1.02) | 0.63 |
| Charlson’s Comorbidity Index | 1.08 (0.91–1.29) | 0.39 |
| ECOG | ||
| 0 | 1.0 | – |
| 1 | 2.41 (0.87–6.64) | 0.089 |
| 2 | 4.00 (1.44–11.12) | 0.008 |
| 3 | 5.57 (1.32–23.52) | 0.020 |
| Smoking (pack-years) | 1.00 (0.99–1.01) | 0.59 |
Abbreviations: HR – hazard ratio. CI – confidence interval. NLR – neutrophil–lymphocyte ratio. SCC – squamous cell carcinoma. NSCLC, NOS – non-small cell lung cancer, not otherwise specified. BED – biologically effective dose. ECOG – Eastern Cooperative Oncology Group.
American Joint Committee on Cancer (AJCC) 7th edition.
Validation of NLR as a pre-treatment prognostic biomarker
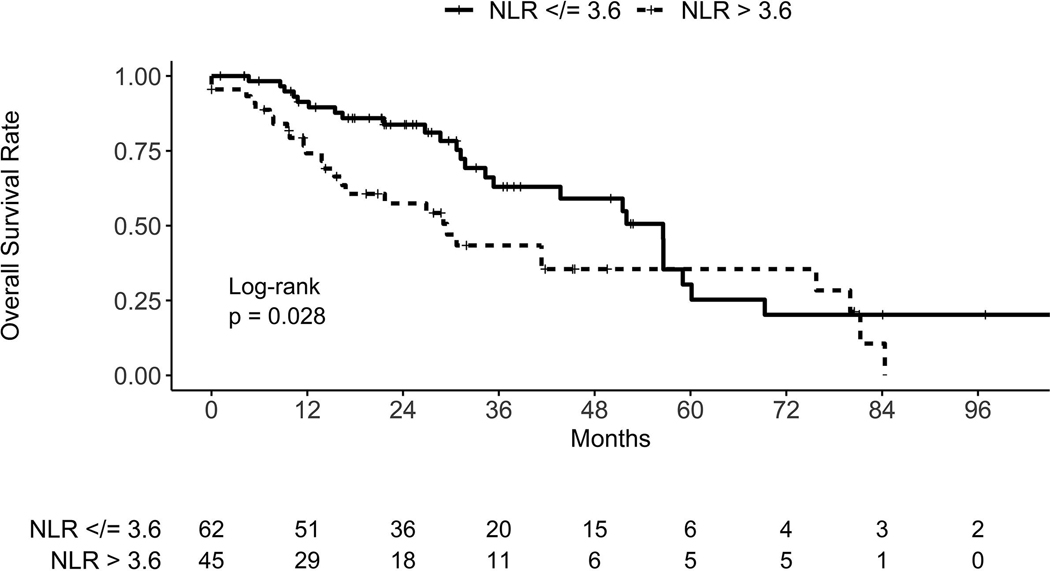
Given the significant association of pre-treatment NLR and mortality in our exploratory analysis, we sought to validate our findings using independent datasets from Massachusetts General Hospital and Rutgers Cancer Institute of New Jersey (see Supplementary Table 1). Due to the smaller sample size from each institution, we pooled their two datasets to provide data on 108 patients (108 tumors). Using pre-treatment NLR data collected from these institutions, we found NLR was significantly associated with mortality when analyzed continuously (HR = 1.06 95% CI 1.03–1.10, p = 0.001) and categorically using the >3.6 cutoff identified in our exploratory analysis (HR = 1.83, 95% CI 1.06–3.16, p = 0.031) (Fig. 2). In addition, after adjusting for age, gender, T-stage, histology, ECOG performance status, smoking, and BEDGy10, NLR remained associated with mortality when analyzed continuously (HR = 1.06, 95% CI 1.02–1.11, p = 0.005) and categorically (HR = 2.43, 95% CI 1.27–4.65, p = 0.007) (Table 3). Once again, there was no significant association of NLR > 3.6 with primary tumor failure (HR = 0.96, 95% CI 0.35–2.66, p = 0.94), regional nodal failure (HR = 1.28, 95% CI 0.55–2.98, p = 0.56), or distant failure (HR = 1.36, 95% CI 0.61–3.0, p = 0.45) (see Supplementary Figs. 4–6). In a subset analysis of the 106 patients with NLR obtained within 3 months (median of 1.2 months) before starting treatment, NLR > 3.6 continued to be associated with inferior overall survival on univariate (HR = 1.92; 95% CI 1.10–3.37; p = 0.02) and multivariate (HR = 2.51; 95% CI 1.31–4.82; p = 0.006) analysis.
Fig. 2.
Kaplan–Meier curves for overall survival in the validation cohort, stratified by neutrophil–lymphocyte ratio (NLR) group.
Table 3.
Cox proportional hazards multivariate analysis of predictors for survival in the validation cohort.*
| Variable | HR (95% CI) | p-value |
|---|---|---|
| NLR | ||
| ≤3.6 | 1.0 | – |
| >3.6 | 2.19 (1.14–4.21) | 0.018 |
| Age | 0.99 (0.95–1.03) | 0.69 |
| Gender | ||
| Male | 1.0 | – |
| Female | 0.37 (0.19–0.72) | 0.003 |
| T-stage† | ||
| T1a | 1.0 | – |
| T1b | 1.18 (0.46–3.01) | 0.73 |
| T2a | 2.25 (0.87–5.84) | 0.10 |
| T2b | 16.77 (2.21–127.51) | 0.006 |
| T3 | 1.40 (0.38–5.20) | 0.62 |
| T4 | – | – |
| Histology | ||
| SCC | 1.0 | – |
| Adenocarcinoma | 1.05 (0.52–2.09) | 0.90 |
| NSCLC, NOS | 1.40 (0.32–6.07) | 0.65 |
| Total BEDGy10 | 1.01 (0.99–1.02) | 0.47 |
| ECOG | ||
| 0 | 1.0 | – |
| 1 | 1.34 (0.59–3.06) | 0.48 |
| 2 | 0.92 (0.33–2.55) | 0.87 |
| 3 | 0.49 (0.08–3.05) | 0.45 |
| Smoking (pack-years) | 1.00 (0.99–1.01) | 0.98 |
Abbreviations: HR – hazard ratio. CI – confidence interval. NLR – neutrophil–lymphocyte ratio. SCC – squamous cell carcinoma. NSCLC, NOS – non-small cell lung cancer, not otherwise specified. BED – biologically effective dose. ECOG – Eastern Cooperative Oncology Group.
American Joint Committee on Cancer (AJCC) 7th edition. T4 excluded due to limited sample size.
Post-treatment NLR as a prognostic biomarker
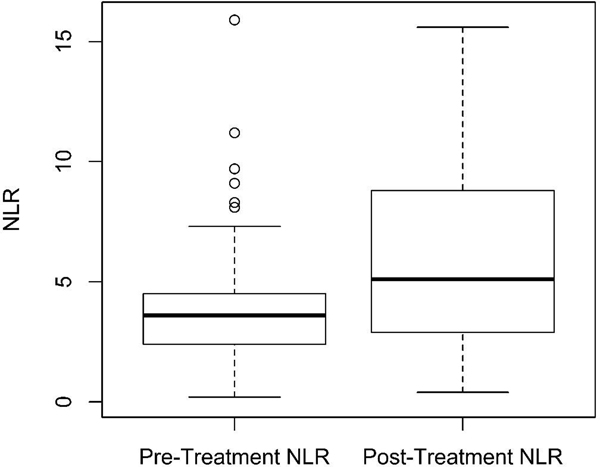
In a secondary analysis of the training cohort, we evaluated the association of post-treatment NLR on survival. Post-treatment NLR was obtained between one to six months after completing SBRT. Of note, fewer patients had post-treatment CBC data, likely due to institutional practice patterns during surveillance. First, we evaluated differences in pre- and post-treatment NLR in patients with both available values (n = 65). Median post-treatment NLR was 5.1 (range 0.4–102.8), obtained at a median of 2.7 months after treatment (range 1.0–6.0 months). Interestingly, we found that there was a significant increase in post-treatment NLR compared to pre-treatment NLR, with post-treatment NLR increasing by a median of 28% (range 87% to 649%) (paired Wilcoxon’s signed-rank test, p < 0.001) (Fig. 3). This increase was predominantly in the group of patients with NLR ≤ 3.6, for whom post-treatment NLR increased by a median 64% (interquartile range [IQR] −7% to 262%), whereas patients with pre-treatment NLR > 3.6 increased by a median of −3% (IQR −25% to 41%) (Wilcoxon’s rank-sum test, p = 0.005). Cox regression univariate analysis showed post-treatment NLR was again significantly associated with mortality (HR = 1.02 [95% CI 1.001–1.04]; p = 0.042) when analyzed as a continuous variable. On Cox multivariate analysis, post-treatment NLR was again associated with overall mortality (HR = 1.03 [95% CI 1.00–1.06]; p = 0.029) when accounting for age, gender, T-stage, histology, ECOG performance status, Charlson’s Comorbidity Index, smoking, and BEDGy10. We did not find a statistically significant association between post-treatment NLR and primary tumor failure (p = 0.56), regional nodal failure (p = 0.74), or distant failure (p = 0.93) (not shown). In a subset analysis of patients with NLR obtained within 2 months after treatment (n = 24) in order to minimize the risk of post-treatment radiation pneumonitis/inflammation contributing to elevated NLR, NLR continued to be associated with overall mortality on univariate (HR = 1.23; 95% CI 1.07–1.41; p = 0.005) and multivariate analysis (HR = 1.54; 95% CI 1.11–2.14; p = 0.01).
Fig. 3.
Boxplot of pre- and post-treatment neutrophil–lymphocyte ratio (NLR). Wilcoxon’s signed rank test, p < 0.001.
Discussion
In this study, we identified pre-treatment NLR is associated with overall survival in patients treated with SBRT for NSCLC. Using a total of 264 patients treated across 3 institutions, we identified NLR > 3.6 is associated with increased mortality. Compared to prior studies, our study is the first to identify a clinically meaningful value of NLR with significant association to overall survival when adjusting for clinically relevant confounders, which are established prognostic factors in patients with early stage NSCLC undergoing SBRT. Furthermore, we validated our cut-point of 3.6 in an independent, multi-institutional cohort of patients. Finally, we identified a significant increase in NLR after SBRT and identified an association of post-treatment NLR with overall survival, both novel findings in the setting of lung SBRT.
Inflammation is an accepted and well-described component of the pathogenesis of cancer [21]. Lymphocytes are believed to play an important role in the natural immune defense against cancer and lymphocyte infiltration of tumors has been shown to correlate with improved survival [22]. Indeed, a relative lymphocytosis prior to starting treatment has been associated with improved outcomes in breast, colorectal and esophageal cancer treated with chemotherapy and radiation [23–24]. On the contrary, neutrophils are hypothesized to promote carcinogenesis. Laboratory studies suggest malignant cells can transform neutrophils into tumor-associated neutrophils (TANs) that promote tumor progression [25]. Additionally, Gooden et al. [22] described increased circulating neutrophils may suppress lymphocytosis, thus eliminating this important arm of host defense and immune surveillance, thereby leading to carcinogenesis.
While there is evidence to suggest an association between NLR and cancer-related response and mortality, there is also evidence suggesting that NLR may be a prognostic biomarker independent of malignancy. Indeed, NLR has been shown to be prognostic in the setting of various benign conditions, such as in the setting of percutaneous coronary intervention or hemodialysis [26–27]. NLR was found to be prospectively associated with all-cause mortality, coronary heart disease, and heart failure in the Jackson Heart Study. The authors concluded NLR may act as a generalized inflammatory marker and, furthermore, the corresponding cutoff portending poor prognosis may vary along with the genetic variability of the different populations that are studied [28].
There are several plausible explanations for our findings. One theory is an elevated NLR reflects the gain of a pro-tumorigenic neutrophilia paired with the loss of anti-neoplastic (or tumor cytotoxic) lymphocytes. A dysfunctional host immune system could lead to inability to mount an anti-tumor response, but also, could put the patient at risk for infectious causes of morbidity and mortality. In the setting of a dysfunctional T cell-mediated immune response, one would predict that higher NLR would predict for inability to properly stimulate tolerant T-cells with immune checkpoint inhibitors such as anti-PD-1 antibodies. As proof of principle, multiple studies have recently concluded that higher NLR is a prognostic and potentially predictive biomarker for poor treatment response and clinical outcomes, not only after chemotherapy, but also after immunotherapy [29–31]. The association of high NLR and inferior outcomes does not appear to be restricted to patients receiving immunotherapy. For example, high NLR has also been shown to be associated with worse progression-free survival and overall survival in advanced NSCLC treated with bevacizumab, an anti-VEGF monoclonal antibody [32]. Taken together, NLR has prognostic and potentially predictive utility in the setting of cancer.
While we found an association of pre-treatment NLR with mortality, we did not find a statistically significant association with disease-control outcomes. This aligns with earlier studies that investigated NLR in early stage NSCLC treated with SBRT [14–17]. Cannon et al. [14] concluded pre-treatment NLR within 3 months of starting SBRT was associated with mortality but not with nonlocal failure. Giuliani et al. [27] reported similar findings in which pre-treatment NLR was independently associated with overall survival but not disease-related death. In contrast, a meta-analysis by Peng et al. [11] showed pre-treatment NLR was associated with treatment response to surgery or chemotherapy in addition to overall survival in NSCLC. Thus, while the association of NLR with mortality appears to be consistent across multiple studies, the impact of NLR on disease-specific outcomes is inconclusive. The results of our study may suggest an underlying propensity for mortality in patients with high NLR that is irrespective of malignancy and the treatment received. However, this does not diminish the utility of NLR as a prognostic biomarker in the setting of lung SBRT, and NLR may be valuable in stratifying appropriateness for treatment and customizing follow-up.
Another novel finding of our study is the association of post-treatment NLR with mortality in the setting of lung SBRT. This latter association has been shown in other disease sites, such as in the treatment of brain metastases with stereotactic radiosurgery [33] as well as locally advanced NSCLC [34–35], and head and neck cancer [36]. Given preclinical evidence that supports the role of radiotherapy in inducing lymphocytic infiltration [37], it is possible that relatively lower post-treatment NLR may be secondary to an anti-tumor lymphocytic response stimulated by radiation, leading to improved survival. Conversely, if the tumor is promoting an immune-tolerant state, it is interesting to speculate that tumor ablation by SBRT is reversing the ability of the tumor to induce immune suppression. Nevertheless, we did not find any correlation between post-treatment NLR and disease control outcomes. Furthermore, we identified higher NLR levels following SBRT, predominantly in the group of patients with pre-treatment NLR < 3.6. Elevation of NLR after radiotherapy has been demonstrated in the setting of radioembolization for liver malignancies [38] and chemoradiation for locally advanced rectal cancer [39]. The significance of this increase in NLR, at a median of 2.7 months after SBRT, is unclear; it is possible this may represent a sustained post-treatment inflammatory state induced by radiotherapy.
Our study is the largest analysis evaluating NLR in the setting of lung SBRT and the only study to validate our pre-treatment NLR cutoff in an independent cohort from separate institutions. However, limitations of this retrospective study include the exclusion of known and unknown concurrent host pro-inflammatory states prior to treatment such as rheumatologic disorders, synchronous malignancies, or medications such as corticosteroids that may have influenced the NLR, as well as a more thorough analysis of factors (e.g. comorbidities) that might influence survival.
In summary, we have found that high pre-treatment NLR is associated with reduced survival in patients undergoing SBRT for early stage NSCLC, notably after accounting for established prognostic variables. This finding was validated in an independent cohort of patients from two other institutions. We also found an association of post-treatment NLR with mortality, a novel finding that merits validation in an independent cohort. Calculating NLR from a peripheral CBC, which is often included in diagnostic workup [40], is a simple, cost-effective and reproducible method to provide additional prognostic data both before and after treatment with SBRT. Integration of this novel host-related prognostic factor into clinical decision-making tools, such as a nomogram to predict the risk of death, may allow for further risk stratification and assist in selecting patients for SBRT (versus other treatment modalities or observation), or by identifying those patients who require closer follow-up after SBRT. Further studies should be conducted using a prospective cohort to evaluate the prognostic significance of NLR in the setting of lung SBRT, as well as pre-clinical studies to better elucidate the role of the innate and adaptive immune systems in contributing to these observations.
Supplementary Material
Acknowledgements
These data were presented in part at the American Society of Radiation Oncology (ASTRO) Annual Meeting 2018 (San Antonio, TX).
Footnotes
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.radonc.2019.01.032.
Conflict of interest disclosures
None of the authors have conflicts of interest to disclose.
References
- [1].Noone AM, Howlader N, Krapcho M, Miller D, Brest A, et al. SEER Cancer Statistics Review, 1975–2015, National Cancer Institute. Bethesda, MD, https://seer.cancer.gov/csr/1975_2015/, based on November 2017 SEER data submission, posted to the SEER web site, April 2018. n.d. [Google Scholar]
- [2].Final Update Summary: Lung Cancer: Screening. U.S. Preventive Services Task Force. July 2015. https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/lung-cancer-screening. [Google Scholar]
- [3].Timmerman R, Paulus R, Galvin J, Michalski J, Straube W, Bradley J, et al. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA 2010;303:1070–6. 10.1001/jama.2010.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Timmerman RD, Paulus R, Pass HI, Gore EM, Edelman MJ, Galvin J, et al. Stereotactic body radiation therapy for operable early-stage lung cancer: findings from the NRG oncology RTOG 0618 Trial. JAMA Oncol 2018;4:1263–6. 10.1001/jamaoncol.2018.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Woodard GA, Jones KD, Jablons DM. Lung cancer staging and prognosis. Cancer Treat Res 2016;170:47–75. 10.1007/978-3-319-40389-2_3. [DOI] [PubMed] [Google Scholar]
- [6].Baine MJ, Verma V, Schonewolf CA, Lin C, Simone CB. Histology significantly affects recurrence and survival following SBRT for early stage non-small cell lung cancer. Lung Cancer 2018;118:20–6. 10.1016/j.lungcan.2018.01.021. [DOI] [PubMed] [Google Scholar]
- [7].Stahl JM, Ross R, Harder EM, Mancini BR, Soulos PR, Finkelstein SE, et al. The effect of biologically effective dose and radiation treatment schedule on overall survival in stage I non-small cell lung cancer patients treated with stereotactic body radiation therapy. Int J Radiat Oncol Biol Phys 2016;96:1011–20. 10.1016/j.ijrobp.2016.08.033. [DOI] [PubMed] [Google Scholar]
- [8].Tas F, Ciftci R, Kilic L, Karabulut S. Age is a prognostic factor affecting survival in lung cancer patients. Oncol Lett 2013;6:1507–13. 10.3892/ol.2013.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Kawaguchi T, Takada M, Kubo A, Matsumura A, Fukai S, Tamura A, et al. Performance status and smoking status are independent favorable prognostic factors for survival in non-small cell lung cancer: a comprehensive analysis of 26,957 patients with NSCLC. J Thorac Oncol 2010;5:620–30. 10.1097/JTO.0b013e3181d2dcd9. [DOI] [PubMed] [Google Scholar]
- [10].Templeton AJ, McNamara MG, Šeruga B, Vera-Badillo FE, Aneja P, Ocaña A, et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst 2014;106. 10.1093/jnci/dju124. [DOI] [PubMed]
- [11].Peng B, Wang Y-H, Liu Y-M, Ma L-X. Prognostic significance of the neutrophil to lymphocyte ratio in patients with non-small cell lung cancer: a systemic review and meta-analysis. Int J Clin Exp Med 2015;8:3098–106. [PMC free article] [PubMed] [Google Scholar]
- [12].Tomita M, Shimizu T, Ayabe T, Nakamura K, Onitsuka T. Elevated preoperative inflammatory markers based on neutrophil-to-lymphocyte ratio and C-reactive protein predict poor survival in resected non-small cell lung cancer. Anticancer Res 2012;32:3535–8. [PubMed] [Google Scholar]
- [13].Sarraf KM, Belcher E, Raevsky E, Nicholson AG, Goldstraw P, Lim E. Neutrophil/lymphocyte ratio and its association with survival after complete resection in non-small cell lung cancer. J Thorac Cardiovasc Surg 2009;137:425–8. 10.1016/j.jtcvs.2008.05.046. [DOI] [PubMed] [Google Scholar]
- [14].Cannon NA, Meyer J, Iyengar P, Ahn C, Westover KD, Choy H, et al. Neutrophil-lymphocyte and platelet-lymphocyte ratios as prognostic factors after stereotactic radiation therapy for early-stage non-small-cell lung cancer. J Thorac Oncol 2015;10:280–5. 10.1097/JTO.0000000000000399. [DOI] [PubMed] [Google Scholar]
- [15].Shaverdian N, Veruttipong D, Wang J, Schaue D, Kupelian P, Lee P. Pretreatment immune parameters predict for overall survival and toxicity in early-stage non-small-cell lung cancer patients treated with stereotactic body radiation therapy. Clin Lung Cancer 2016;17:39–46. 10.1016/j.cllc.2015.07.007. [DOI] [PubMed] [Google Scholar]
- [16].Giuliani M, Sampson LR, Wong O, Gay J, Le LW, Cho BCJ, et al. Prognostic value of pretreatment circulating neutrophils, monocytes, and lymphocytes on outcomes in lung stereotactic body radiotherapy. Curr Oncol 2016;23:e362–8. 10.3747/co.23.3051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Luo H, Ge H, Cui Y, Zhang J, Fan R, Zheng A, et al. Systemic inflammation biomarkers predict survival in patients of early stage non-small cell lung cancer treated with stereotactic ablative radiotherapy - a single center experience. J Cancer 2018;9:182–8. 10.7150/jca.21703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Haasbeek CJA, Lagerwaard FJ, Slotman BJ, Senan S. Outcomes of stereotactic ablative radiotherapy for centrally located early-stage lung cancer. Journal of Thoracic Oncology 2011;6:2036–43. 10.1097/JTO.0b013e31822e71d8. [DOI] [PubMed] [Google Scholar]
- [19].Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol 2010;17:1471–4. 10.1245/s10434-010-0985-4. [DOI] [PubMed] [Google Scholar]
- [20].Radiation Therapy Oncology Group 0813. Seamless phase I/II study of stereotactic lung radiotherapy (SBRT) for early stage, centrally located, nonsmall cell lung cancer (NSCLC) in medically inoperable patients. Version date 6/8/2015. https://www.rtog.org/ClinicalTrials/ProtocolTable/StudyDetails.aspx?study=0813. n.d.
- [21].Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature 2008;454:436–44. 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- [22].Gooden MJM, de Bock GH, Leffers N, Daemen T, Nijman HW. The prognostic influence of tumour-infiltrating lymphocytes in cancer: a systematic review with meta-analysis. Br J Cancer 2011;105:93–103. 10.1038/bjc.2011.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Lee HJ, Seo J-Y, Ahn J-H, Ahn S-H, Gong G. Tumor-associated lymphocytes predict response to neoadjuvant chemotherapy in breast cancer patients. J Breast Cancer 2013;16:32–9. 10.4048/jbc.2013.16.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Jung SW, Park IJ, Oh SH, Yeom S-S, Lee JL, Yoon YS, et al. Association of immunologic markers from complete blood counts with the response to preoperative chemoradiotherapy and prognosis in locally advanced rectal cancer. Oncotarget 2017;8:59757–65. 10.18632/oncotarget.15760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Wu Y, Zhao Q, Peng C, Sun L, Li X-F, Kuang D-M. Neutrophils promote motility of cancer cells via a hyaluronan-mediated TLR4/PI3K activation loop. J Pathol 2011;225:438–47. 10.1002/path.2947. [DOI] [PubMed] [Google Scholar]
- [26].Duffy BK, Gurm HS, Rajagopal V, Gupta R, Ellis SG, Bhatt DL. Usefulness of an elevated neutrophil to lymphocyte ratio in predicting long-term mortality after percutaneous coronary intervention. Am J Cardiol 2006;97:993–6. 10.1016/j.amjcard.2005.10.034. [DOI] [PubMed] [Google Scholar]
- [27].Catabay C, Obi Y, Streja E, Soohoo M, Park C, Rhee CM, et al. Lymphocyte cell ratios and mortality among incident hemodialysis patients. AJN 2017;46:408–16. 10.1159/000484177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Kim S, Eliot M, Koestler DC, Wu W-C, Kelsey KT. Association of neutrophil-to-lymphocyte ratio with mortality and cardiovascular disease in the Jackson heart study and modification by the duffy antigen variant. JAMA Cardiol 2018;3:455–62. 10.1001/jamacardio.2018.1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Russo A, Franchina T, Ricciardi GRR, Battaglia A, Scimone A, Berenato R, et al. Baseline neutrophilia, derived neutrophil-to-lymphocyte ratio (dNLR), platelet-to-lymphocyte ratio (PLR), and outcome in non small cell lung cancer (NSCLC) treated with Nivolumab or Docetaxel. J Cell Physiol 2018;233:6337–43. 10.1002/jcp.26609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Bagley SJ, Kothari S, Aggarwal C, Bauml JM, Alley EW, Evans TL, et al. Pretreatment neutrophil-to-lymphocyte ratio as a marker of outcomes in nivolumab-treated patients with advanced non-small-cell lung cancer. Lung Cancer 2017;106:1–7. 10.1016/j.lungcan.2017.01.013. [DOI] [PubMed] [Google Scholar]
- [31].Diem S, Schmid S, Krapf M, Flatz L, Born D, Jochum W, et al. Neutrophil-to-Lymphocyte ratio (NLR) and Platelet-to-Lymphocyte ratio (PLR) as prognostic markers in patients with non-small cell lung cancer (NSCLC) treated with nivolumab. Lung Cancer 2017;111:176–81. 10.1016/j.lungcan.2017.07.024. [DOI] [PubMed] [Google Scholar]
- [32].Botta C, Barbieri V, Ciliberto D, Rossi A, Rocco D, Addeo R, et al. Systemic inflammatory status at baseline predicts bevacizumab benefit in advanced non-small cell lung cancer patients. Cancer Biol Ther 2013;14:469–75. 10.4161/cbt.24425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Chowdhary M, Switchenko JM, Press RH, Jhaveri J, Buchwald ZS, Blumenfeld PA, et al. Post-treatment neutrophil-to-lymphocyte ratio predicts for overall survival in brain metastases treated with stereotactic radiosurgery. J Neurooncol 2018;139:689–97. 10.1007/s11060-018-2914-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Bazan JG, Barney C, Scoville N, Haglund KE, Grecula JC, Welliver MX, et al. Increased posttreatment neutrophil-to-lymphocyte ratio is an independent prognostic marker for worse overall survival in patients receiving concurrent chemoradiation for lung cancer. Int J Radiat Oncol Biol Phys 2016;96:E479. 10.1016/j.ijrobp.2016.06.1833. [DOI] [Google Scholar]
- [35].Suh KJ, Kim SH, Kim YJ, Kim M, Keam B, Kim TM, et al. Post-treatment neutrophil-to-lymphocyte ratio at week 6 is prognostic in patients with advanced non-small cell lung cancers treated with anti-PD-1 antibody. Cancer Immunol Immunother 2018;67:459–70. 10.1007/s00262-017-2092-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Kim DY, Kim IS, Park SG, Kim H, Choi YJ, Seol YM. Prognostic value of posttreatment neutrophil–lymphocyte ratio in head and neck squamous cell carcinoma treated by chemoradiotherapy. Auris Nasus Larynx 2017;44:199–204. 10.1016/j.anl.2016.05.013. [DOI] [PubMed] [Google Scholar]
- [37].Sharabi AB, Nirschl CJ, Kochel CM, Nirschl TR, Francica BJ, Velarde E, et al. Stereotactic radiation therapy augments antigen-specific PD-1-mediated antitumor immune responses via cross-presentation of tumor antigen. Cancer Immunol Res 2015;3:345–55. 10.1158/2326-6066.CIR-14-0196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].D’Emic N, Engelman A, Molitoris J, Hanlon A, Sharma NK, Moeslein FM, et al. Prognostic significance of neutrophil-lymphocyte ratio and platelet-lymphocyte ratio in patients treated with selective internal radiation therapy. J Gastrointest Oncol 2016;7:269–77. 10.3978/j.issn.2078-6891.2015.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Sung S, Son SH, Park EY, Kay CS. Prognosis of locally advanced rectal cancer can be predicted more accurately using pre- and post-chemoradiotherapy neutrophil-lymphocyte ratios in patients who received preoperative chemoradiotherapy. PLoS ONE 2017;2:e0173955. 10.1371/journal.pone.0173955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Silvestri GA, Gonzalez AV, Jantz MA, Margolis ML, Gould MK, Tanoue LT, et al. Methods for Staging non-small cell lung cancer: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2013;143:e211S–50S. 10.1378/chest.12-2355. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.