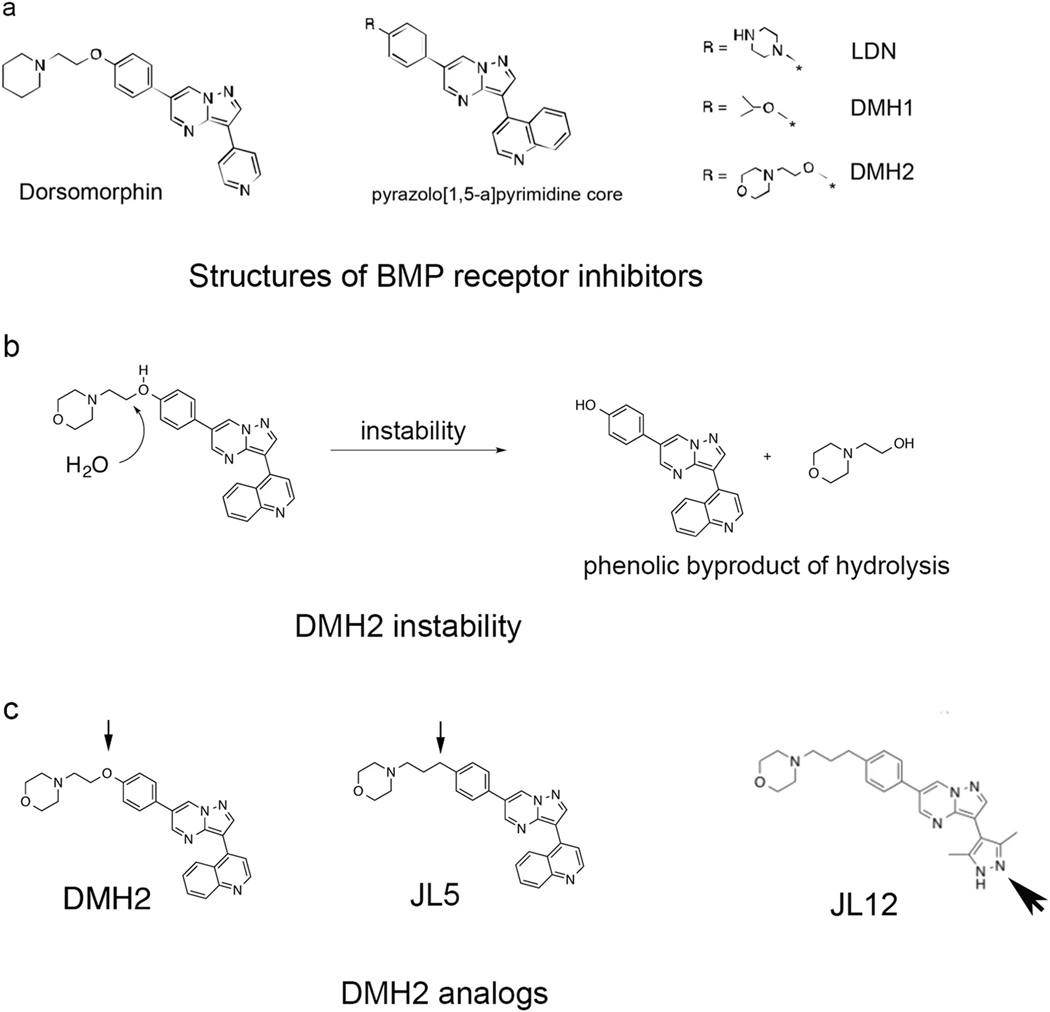
Fig. 2.
A carbon for oxygen substitution to DMH2 results in the formation of JL5. a Structures of Dorsomorphin analogs, which were derived from the same pyrazolo[1,5-a] pyrimidine core. BMP inhibitors DMH2, DMH1, and LDN differ in the substitutions made at the R position of the pyrazolo[1,5-a] pyrimidine core. b DMH2 was found to be chemically and metabolically unstable. LCMS of a sample of stored DMH2 for 4 months revealed the phenolic byproduct due to morpholine side-chain hydrolysis. c Structure of DMH2 analogs JL5 and the inactive analog JL12. The small arrowhead shows the carbon substitution for oxygen in the DMH2 side chain to create JL5. The large arrowhead shows the imidapyrazole substitution made to JL5 to create JL12