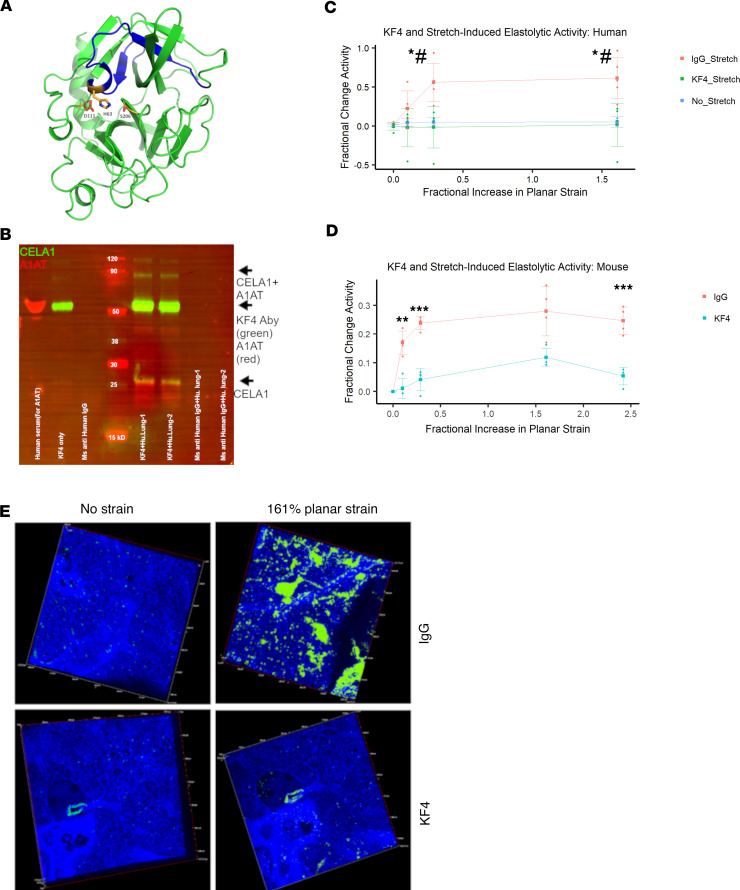
Figure 8. CELA1 and stretch-inducible elastase activity in human lung.
(A) The 3D model of human CELA1 retrieved from the Swiss-Model repository (https://swissmodel.expasy.org/repository; ID: Q9UNI1) with the epitope detected by the KF4 antibody highlighted in blue. The 3 amino acids of the catalytic triad (H63, D111, and S206) are shown. The histidine of the CELA1 catalytic triad is within the KF4 epitope. (B) Western blot of a human lung immunoprecipitation (IP) experiment using 2 human organ donor lung specimens. Lanes 1 and 2 are eluates from IP with KF4. Lanes 3 and 4 are eluates from IP with a mouse anti–human IgG antibody. Lanes 5 and 6 are eluates from IP with a polyclonal guinea pig anti-CELA1 antibody. For immunostaining, KF4 and anti–human α-1 antitrypsin (anti-AAT) antibodies were used as primary antibodies and detected with anti-mouse (green) and anti-rabbit (red) secondary antibodies, respectively. The native 28 kDa CELA1 band is detected (green arrow) and a complex of CELA1 and AAT is detected at approximately 60 kDa (yellow arrow). (C) Five human lung sections were subjected to biaxial stretch in the presence of IgG or KF4 or incubated for equivalent times without stretch in the presence of elastin in situ zymography substrate. Stretch-inducible elastase activity was detected only in the IgG group. Comparisons at each time point are by ANOVA (P < 0.05) with post hoc Tukey’s test P values shown. *P < 0.05 KF4 vs. IgG, #P < 0.05 KF4 vs. unstretched. (D) Stretch-inducible lung elastase activity in mouse lung sections showing approximately 80% reduction in activity at all points. **P < 0.01, ***P < 0.001 by 2-tailed Welch’s t test. (E) Representative images showing elastase activity in mouse lung sections subjected to biaxial stretch with IgG and KF4 treatments.