Abstract
Rice is an important food crop, while it is severely affected by drought stress. Viewing this point in mind we conducted an experiment to see the physiological responses and yield potential of selected rice genotypes under inadequate moisture condition. Two soil moisture condition: one is sufficient moisture condition (control; 95–100% field capacity (FC)) and another is deficit moisture condition (moisture stress; 40–45% FC) with five replications was maintained. Six drought tolerant one susceptible genotype and one standard check variety were used. Results revealed that tolerant genotypes BU Acc 37 and BU Acc 32 showed the highest RWC, WRC, rate of photosynthesis, conductance of stomata, transpiration rate, total chlorophyll content, proline and soluble sugar content, while susceptible genotype BU Acc 5 showed the lowest value of those parameters during water stress condition. In contrast, the lowest WSD, WUC, accumulation of H2O2 and malondialdehyde were noticed in tolerant genotypes BU Acc 37 and BU Acc 32, whereas those were the highest in susceptible genotype BU Acc 5 under deficit moisture condition. Tolerant genotype BU Acc 37 and BU Acc 32 also showed the higher antioxidant enzyme activity than the susceptible genotype BU Acc 5. Regardless of genotypes, yield contributing characteristics and yield were severely affected by deficit water stress. However, tolerant genotype BU Acc 37 showed the highest grain yield per hill, while susceptible genotype BU Acc 5 showed the lowest grain yield per hill. Hence, better accumulation ability of osmoprotectants, and the higher activity of antioxidant enzymes in the tolerant genotypes reduce the oxidative stress, enhance water relation and gas exchange attributes, and protect the yield reduction of rice.
Keywords: Moisture stress, Gas exchange characteristics, Proline, Reactive oxygen species, Antioxidant, Rice yield
1. Introduction
Rice uses as a staple meal for nearly half of the population of the world (Urmi et al., 2023, Saha et al., 2019). Rice is the authentic source of minerals and fiber, and contributes 20 % of the total energy and 15 % of the world population's protein requirements. Asian countries produce and consume about ninety-two percent of the total rice of the globe (Fahad et al., 2017). Annual production of rice in Asia is 516 million tonnes which is cultivated on 135 million ha (Saha et al., 2019). Bangladesh ranked four in the globe based on rice production. Nearly 170 million people of Bangladesh consume rice as staple food. Based on increasing rate, by the year of 2050, population of Bangladesh may reach 238 million (Shelley et al., 2016). Population of the world is also increasing overtime, and by 2050 it is expected to reach about 9.1 billion, though rising of agricultural production is not same (Molotoks et al., 2021). Therefore, the world agriculture production ought to be redoubled by 60–110 % for an additional pair of 2.3 billion people within year 2050. On the other hand, impacts of different biotic and abiotic stresses on agricultural production increase over time (Islam et al., 2023). The widespread abiotic stresses whose are responsible for yield reduction are drought, salinity, flood, cold, and heat. Among these stresses, drought is considered as the most hazardous stress which severely collapses the production of agricultural (Urmi et al., 2023). Drought exaggerated about 40 % of the world’s population while near 700 million people is at-risk of being displaced as a result of drought by 2023 (Urmi et al., 2023).
Water scarcity severely reduces the growth, physiology and yield of rice. Drought affects morphological parameters of rice by reducing germination percentage, stem elongation, plant biomass, tiller number per hill and leaves per hill (Islam et al., 2018). Under drought, the major physiological alterations are reduction in relative water content (RWC), photosynthesis, transpiration, conductance of stomatal and photosynthetic pigments content (Tamanna et al., 2023). Growth and production of crop depends on photosynthesis which is affected by deficit soil moisture (Yang et al., 2019). Photosynthesis of rice leaf is the result of captured solar energy, up-taking of atmospheric carbon dioxide (CO2), and the mechanisms of leaf gas exchange (Yang et al., 2019). Reduction of photosynthesis of crops depends on decline of turgor pressure, lower stomatal conductance, decrease in gas exchange and CO2 assimilation of leaf (Zhu et al., 2020, Gupta et al., 2020). Drought stress induces shortening of stomatal pores, transpiration rate along with uptake of reduced CO2 and reduces photosynthesis (Souza et al., 2013). Plant absorbs light and transfers light energy to photosystem through leaf chlorophyll (Rahdari et al., 2012). Water scarcity enhances the pigment photooxidation and chlorophyll degradation and reduces the leaf chlorophyll content (Anjum et al., 2011). Deficit water inhibit canopy development and suppress photosynthesis which leads to less accumulation of dry matter finally reduces the growth and yield of crops (Tamanna et al., 2023).
Higher production of reactive oxygen species (ROS) under inadequate moisture condition enhances lipid peroxidation and raises the production of malondialdehyde (MDA). Malondialdehyde is an index of drought induces oxidative damage caused (Urmi et al., 2023). Leaves senescence occur through breakdown of photosynthetic pigments under excessive accumulation of ROS (Urmi et al., 2023, Hussain et al., 2018, Noctor et al., 2018). Through signal transduction pathway plants modify their physiological and biochemical attributes for their existence under stress (Urmi et al., 2023, Siddiqui et al., 2019). Thus, plant shows adaptive responses to stress by producing different osmolites, especially proline and soluble sugar. Plant physiology, membranes integrity and stability of enzymes are regulated by those osmoprotectants (Kurepin et al., 2017, Dien et al., 2019). Therefore, accumulation of proline, soluble sugar as well as activities of antioxidant enzymes can be used as an indicator of stress tolerance of crops (Urmi et al., 2023, Tamanna et al., 2023).
At present, suitable drought tolerant rice cultivar is essential for the expansion of rice production in drought prone areas of Bangladesh as well as of the globe. Fluctuation of hydrological level under this adverse climatic condition is one of the major limiting factors of agricultural production. In contrast, food demand of the globe increases due to increasing population. In this situation, it is essential to use those less favorable lands for crop production. Therefore, it is essential to screen crop varieties tolerance to drought stress. It is reported that physiological characteristics such as RWC, LWP, rate of photosynthesis, conductance of stomata, photosynthetic pigmant, proline, sugar content, ROS, MDA and antioxidant enzymes activities are closely correlated with drought stress tolerance of rice and those characteristics can be used as criteria for selection for drought stress (Li et al., 2016, Panda et al., 2021). In these circumstances, characterization of rice physiology in the moisture stress environment is crucial for development of moisture stress tolerance rice variety. Understanding physiology of rice under deficit soil moisture conditions will be useful in breeding programs for developing a drought tolerant rice variety which is essential for drought prone ecosystem of the globe. We speculate that better physiological characteristics and maximum grain yield may be found in rice genotypes which are tolerance to moisture stress.
2. Materials and methods
2.1. Plant materials and treatments
Pre-evaluated six drought tolerant (BU Acc 7, BU Acc 12, BU Acc 24, BU Acc 25, BU Acc 32, BU Acc 37) one susceptible genotype (BU Acc 5) and one standard check variety (BRRI dhan43) of rice were used. The treatment variables of this experiment were eight rice genotypes and two sets of moisture regime: one is sufficient moisture condition (control; 95 to 100 % field capacity (FC)) and another is deficit moisture condition (moisture stress; 40 to 45 % FC). Vinyl house was used to conducted this experiment. Five replications and factorial completely randomized design (CRD) was maintained in this experiment.
2.2. Pot preparation and application of fertilizer
The pot was filled with 13 kg soil which was mixed with cow dung at 1:0.25 ratios. Bulk density and particle density of the silty clay loam pot soil were 1.36 g/cc and 2.61 g/cc, respectively. Soil pH was 5.94. Organic carbon and total nitrogen were 0.97 and 0.093 %, respectively. Available P, exchangeable K and available S were 18.87 mg kg−1 soil, 0.127 meq/100 g soil and 20.91 mg kg−1 soil, respectively. Field capacity was 30.55 % vol/vol. Fertilizers at the rate of 1.30 g of urea, 0.51 g of triple super phosphate (TSP), 0.70 g of muriate of potash (MoP), 0.19 g of gypsum and 0.081 g of zinc sulphate were incorporated in each pot according to BRRI (2020).
2.3. Sowing of seed and treatment imposition
Distilled water was used for washing of the surface sterilized seeds. Soil containing plastic tray were used for germination. Rice seedlings of twenty-one-day old were transferred to plastic pots. Treatments were imposed at 28 days after transplantation (DAT). Two sets of moisture regime: one is sufficient moisture condition (control; 95–100 % FC) and another is deficit moisture condition (moisture stress; 40–45 % FC) were maintained. Soil moisture was monitored and maintained according to Urmi et al. (2023). Irrigation water was applied by measuring cylinder. Soil moisture meter (Stevens, Field POGO, Portland, Oregon, USA) was used to assess the field capacity of the soil. Plant samples were collected after four (4) weeks of treatment imposition from five replications for all physiological data and antioxidant enzyme activity.
2.4. Assessment of water relation parameters
Different water relation parameters were calculated four weeks after treatment imposition as describe Tamanna et al. (2023).
2.5. Estimation of gas exchange characteristics
Four weeks after treatment imposition, portable photosynthetic system Li-COR, 6400 (Li-COR, Lincon, NE, USA) was used for taking data of photosynthesis (Pn), conductance of stomata (gs), and rate of transpiration (Tr) as described by Urmi et al. (2023). Fully expanded uppermost leaves of each variety of all the treatments were used in gas exchange measurements. All measurements were taken in a sunny day.
2.6. Estimation of photosynthetic pigment
Spectrophotometer (double-beam) was used to determine photosynthetic pigment like chlorophyll content four weeks after application of treatment as described Urmi et al. (2023). Chlorophyll content was determined based on fresh weight basis extracting with 80 % acetone.
2.7. Determination of osmoprotectants
Different osmoprotectants such as proline content and soluble sugar were measured according to Urmi et al. (2023).
2.8. Oxidative stress assessment
Hydrogen peroxide (H2O2) was determined five times according to the method of Islam et al. (2023) after four weeks of treatment imposition. A total of 500 mg fresh leaf was macerated with 0.1 % trichloroacetic acid (TCA), and the homogenate centrifuged at 10,000 × g for 10 min. A total of 0.75 mL of 100 mM potassium phosphate buffer (pH 7.0) and 1Mpotassium iodide were mixed with supernatant (0.75 mL). Spectrophotometer (Shimadzu, UV-1201; 1, Nishinokyo Kuwabara-cho, Nakagyo-ku, Kyoto 604-8511, Japan) was used to measure at 390 nm.
Malondialdehyde (MDA) was estimated five times according to the method of Islam et al. (2023) after four weeks of treatment imposition. A total of 500 mg of fresh leaf were ground in 0.1 % trichloroacetic acid (TCA) and centrifuged at 15,000g for 10 min. Then, 1 mL of supernatant was added to 4 mL of thiobarbituric acid (TBA) (prepared in 20 % TBA) and boiled at 100 °C for 30 min. The reaction mixture was terminated in an ice bath followed by centrifugation at 15,000g for 10 min. Finally, the colored supernatants absorbance was measured at 530 nm and 600 nm using spectrophotometer (Shimadzu, UV-1201; 1, Nishinokyo Kuwabara-cho, Nakagyo-ku, Kyoto 604–8511, Japan).
2.9. Estimation of antioxidant enzymatic activity
Potassium phosphate buffer having polyvinyl pyrrolidone (1 %) with pH 7.0 was used for homogeneous of fresh leaf tissue in pestle and mortar under deep-freezer-cooled condition. The homogenates were centrifuged at 4 °C for 30 min at 12,000g and the supernatant was collected. These supernatant was used for determination of catalase (CAT, EC: 1.11.1.6), ascorbate peroxidase (APX, EC: 1.11.1.11) and superoxide dismutase (SOD, EC: 1.15.1.1) activity. CAT and APX activity were measured according to Islam et al. (2023) and SOD activity was measured according to Urmi et al. (2023).
2.10. Yield assessment
Different rice genotypes were harvested 110–130 days after transplanting. Yield data such as plant height, total tiller per hill, effective tiller per hill, spike length, filled grain per panicle, thousand grain weight and grain yield per plant were recorded from each pot. Plant height was measured from the surface level of the soil to the tip of the longest spike of the plant.
2.11. Statistical analysis
Statistix version 10 software was used for statistical analysis of the collected data. The observed data were statistically analyzed using ‘Statistix version 10′ software. ANOVA technique was applied to scrutinize the collected data. Mean value was compered by Tukey’s test at 5 % level of significant.
3. Results
3.1. Water relation characteristics of rice
3.1.1. Relative water content
In this study, both the year, relative water content (RWC) of rice genotypes was considerably affected by deficit moisture stress (Table 1). However, under moisture stress condition, the highest RWC was found in genotype BU Acc 37 (1st year: 76.4 %; 2nd year: 75.2 %) followed by genotype BU Acc 32, BRRI dhan 43 and the lowest RWC content was observed in BU Acc 5 (1st year: 61.4 %; 2nd year: 60.7 %). The percent decrease of RWC is an important indicator of stress tolerance. Similar kinds of decrease in RWC were found in rice genotypes in both years. However, during both years, the lowest percent decrease over the control was found in genotype BU Acc 37 (1st year: 14.4 %; 2nd year: 17.8 %) next genotype BU Acc 32, while the genotype BU Acc 5 showed the highest percent decrease (1st year: 30.0 % 2nd year: 32.0 %).
Table 1.
Interaction effects of two moisture levels and different rice genotypes on water relation characteristics of rice.
| Moisture levels | Genotypes | RWC (%) |
WSD (%) |
WRC |
WUC |
||||
|---|---|---|---|---|---|---|---|---|---|
| Year I | Year II | Year I | Year II | Year I | Year II | Year I | Year II | ||
| Control | BU Acc 5 | 87.7 ± 2.62 b | 89.3 ± 3.25 b | 7.6 ± 0.41 f | 7.4 ± 0.25g | 4.81 ± 0.25 a | 4.93 ± 0.31 a | 0.36 ± 0.03 h | 0.42 ± 0.05 g |
| BU Acc 7 | 91.2 ± 4.33 a | 90.4 ± 3.15 ab | 7.8 ± 0.36 f | 7.2 ± 0.31 g | 4.88 ± 0.41 a | 4.89 ± 0.25 ab | 0.35 ± 0.02 h | 0.39 ± 0.03 g | |
| BU Acc 12 | 87.6 ± 3.46 b | 88.1 ± 2.62 bc | 7.8 ± 0.51 f | 7.5 ± 0.45 g | 4.69 ± 0.35 b | 4.96 ± 0.22 a | 0.37 ± 0.02 h | 0.41 ± 0.03 g | |
| BU Acc 24 | 89.5 ± 3.61 ab | 88.6 ± 2.55 bc | 7.5 ± 0.22 f | 7.3 ± 0.25 g | 4.85 ± 0.45 a | 4.91 ± 0.25 a | 0.36 ± 0.06 h | 0.44 ± 0.01 g | |
| BU Acc 25 | 87.7 ± 2.81 b | 90.4 ± 3.11 ab | 7.6 ± 0.71 f | 7.1 ± 0.20 gh | 4.78 ± 0.30 ab | 4.96 ± 0.30 a | 0.36 ± 0.04 h | 0.41 ± 0.03 g | |
| BU Acc 32 | 88.6 ± 2.64 b | 90.3 ± 3.25 ab | 7.8 ± 0.35 f | 7.5 ± 0.30 g | 4.83 ± 0.45 a | 4.93 ± 0.30 a | 0.37 ± 0.03 h | 0.40 ± 0.02 g | |
| BU Acc 37 | 89.3 ± 4.52 ab | 91.5 ± 2.16 a | 7.6 ± 0.26 f | 7.3 ± 0.41 g | 4.87 ± 0.40 a | 4.92 ± 0.30 a | 0.36 ± 0.03 h | 0.41 ± 0.03 g | |
| BRRI dhan43 | 91.6 ± 3.67 a | 91.5 ± 1.65 a | 7.4 ± 0.52 f | 7.3 ± 0.26 g | 4.81 ± 0.32 a | 4.92 ± 0.25 a | 0.35 ± 0.03 h | 0.42 ± 0.02 g | |
| Moisture stress |
BU Acc 5 | 61.4 ± 1.62 g | 60.7 ± 2.55 i | 22.5 ± 1.16 a | 21.3 ± 0.91 a | 3.29 ± 0.36 g | 3.13 ± 0.22 h | 0.895 ± 0.07 a | 0.891 ± 0.06 a |
| BU Acc 7 | 65.3 ± 2.05 f | 63.4 ± 3.12 h | 14.4 ± 0.82 b | 14.1 ± 0.58 b | 3.51 ± 0.41 f | 3.51 ± 0.21 g | 0.813 ± 0.07 b | 0.811 ± 0.05 b | |
| BU Acc 12 | 62.4 ± 1.55 g | 63.5 ± 3.27 h | 14.1 ± 0.72 b | 14.3 ± 0.65 b | 3.76 ± 0.20 ef | 3.56 ± 0.25 g | 0.761 ± 0.06 c | 0.803 ± 0.05 b | |
| BU Acc 24 | 67.8 ± 1.48 e | 68.2 ± 1.62 fg | 13.3 ± 0.61 bcd | 12.9 ± 0.52 c | 3.88 ± 0.34 e | 3.71 ± 0.32 f | 0.705 ± 0.07 d | 0.711 ± 0.06 c | |
| BU Acc 25 | 70.5 ± 1.06 d | 69.6 ± 2.52 f | 12.7 ± 0.34 cd | 12.3 ± 0.35 cd | 4.12 ± 0.21 d | 3.96 ± 0.25 e | 0.611 ± 0.06 e | 0.625 ± 0.02 d | |
| BU Acc 32 | 71.3 ± 2.25 d | 72.6 ± 2.31 e | 11.9 ± 0.62 de | 11.7 ± 0.35 e | 4.36 ± 0.18 c | 4.21 ± 0.35 d | 0.581 ± 0.04 f | 0.593 ± 0.05 e | |
| BU Acc 37 | 76.4 ± 2.71 c | 75.2 ± 2.58 d | 10.7 ± 0.66 e | 10.3 ± 0.41 f | 4.41 ± 0.27 c | 4.35 ± 0.25 c | 0.551 ± 0.04 g | 0.561 ± 0.06 f | |
| BRRI dhan43 | 70.0 ± 2.08 de | 71.4 ± 1.96 e | 12.6 ± 0.71 d | 11.5 ± 0.25 e | 4.35 ± 0.35 c | 4.23 ± 0.30 d | 0.595 ± 0.05 e | 0.591 ± 0.03 e | |
Significantly different mean values of individual column are shown in dissimilar letters based on Tukey’s test at 5 % level of significance. The data of five replicates ± SE are shown. RWC: Relative water content; WSD: Water saturation deficit; WRC: Water retention capacity; WUC: Water uptake capacity. SE: standard error.
3.1.2. Water saturation deficit
In this study, regardless of genotypes, deficit moisture stress markedly influenced the water saturation deficit (WSD) of the rice plants (Table 1). However, under moisture stress, the lowest WSD was found in genotype BU Acc 37 (1st year: 10.7 %; 2nd year: 10.3 %) next genotype BU Acc 32 (1st year: 11.9 %; 2nd year: 11.7 %) whereas genotype BU Acc 5 showed the highest WSD (1st year: 22.5 %; 2nd year: 21.3 %). Genotype BU Acc 37 also showed the lowest percent increase over control (1st year: 40.8 %; 2nd year: 40.8 %), while the genotype BU Acc 5 showed the highest (1st year: 196.1 %; 2nd year: 195.1 %).
3.1.3. Water retention capacity
Under control condition, all rice genotypes showed statistically similar water retention capacity (WRC) (Table 1). However, under deficit soil moisture, genotype BU Acc 37 showed the highest WRC (1st year: 4.41; 2nd year: 4.35) after that genotype BU Acc 32, while the lowest WRC was observed in BU Acc 5 (1st year: 3.29; 2nd year: 3.13). Percent decrease over control was the lowest in BU Acc 37 (1st year: 9.4 %; 2nd year: 11.6 %) next genotype BU Acc 32, whereas genotype BU Acc 5 showed the highest (1st year: 31.6 %; 2nd year: 36.5 %).
3.1.4. Water uptake capacity
In this study, water uptake capacity (WUC) of rice genotypes was markedly increased due to deficit water stress (Table 1). At control, statistically identical results were found among the rice genotypes during both years. However, under deficit moisture stress, genotype BU Acc 37 showed the lowest WUC (1st year: 0.551; 2nd year: 0.561) next genotype BU Acc 32, while the genotype BU Acc 5 showed the highest WUC (1st year: 0.895; 2nd year: 0.891). Genotype BU Acc 37 also showed the lowest percent increase over control (1st year: 53.1 %; 2nd year: 36.8 %) next BU Acc 32, whereas genotype BU Acc 5 showed the highest (1st year: 148.6 % 2nd year: 112.1 %). These results revealed that WUC was less in drought tolerant genotypes BU Acc 37 and BU Acc 32 and high in drought sensitive genotype BU Acc 5 under deficit soil moisture.
3.2. Gas exchange activities of rice
3.2.1. Rate of photosynthesis
In this experiment, regardless of genotypes, deficit moisture stress markedly affected the photosynthetic rate (Pn) of rice genotypes during both year (Table 2). Under deficit moisture condition, genotype BU Acc 37 showed the highest Pn value (1st year: 14.9 µmol m−2 s−1; 2nd year: 14.8 µmol m−2 s−1) followed by BU Acc 32, whereas genotype BU Acc 5 showed the lowest Pn value (1st year: 6.2 µmol m−2 s−1; 2nd year: 6.4 µmol m−2 s−1). The lowest percent decrease over control of Pn was observed in genotype BU Acc 37 (1st year: 19.5 %; 2nd year: 18.2 %) next BU Acc 32, while BU Acc 5 showed the highest (1st year: 59.2 %; 2nd year: 57.0 %). As the genotype BU Acc 37 and BU Acc 32 showed lower reduction rate of Pn under drought stress, they might be considered as drought tolerant genotypes.
Table 2.
Effects of interaction between two moisture levels and different rice genotypes on gas exchange attributes of rice.
| Moisture levels | Genotypes | Rate of photosynthesis (µmol/ m/ s) |
Conductance of stomatal (mmol/ m/ s) |
Rate of transpiration (mmol/ m /s) |
Content of total chlorophyll |
||||
|---|---|---|---|---|---|---|---|---|---|
| Year I | Year II | Year I | Year II | Year I | Year II | Year I | Year II | ||
| Control | BU Acc 5 | 15.2 ± 0.68 e | 14.9 ± 1.02 f | 0.310 ± 0.03 ab | 0.311 ± 0.05 a | 5.01 ± 0.35 a | 4.97 ± 0.46 a | 3.15 ± 0.15 bc | 3.18 ± 0.25 b |
| BU Acc 7 | 16.8 ± 0.51 cd | 15.8 ± 0.82 e | 0.297 ± 0.02 c | 0.304 ± 0.04 ab | 4.92 ± 0.35 a | 4.95 ± 0.55 a | 3.20 ± 0.20 bc | 3.18 ± 0.30 b | |
| BU Acc 12 | 16.5 ± 0.81 d | 15.7 ± 0.91 e | 0.317 ± 0.03 a | 0.316 ± 0.05 a | 4.99 ± 0.31 a | 5.01 ± 0.71 a | 3.55 ± 0.25 ab | 3.49 ± 0.30 a | |
| BU Acc 24 | 17.4 ± 0.75 bc | 17.5 ± 1.05 c | 0.293 ± 0.03 cd | 0.307 ± 0.05 ab | 5.12 ± 0.40 a | 5.06 ± 0.45 a | 3.23 ± 0.20 bc | 3.31 ± 0.21 ab | |
| BU Acc 25 | 18.1 ± 0.58 ab | 17.8 ± 1.11 ab | 0.307 ± 0.03 b | 0.311 ± 0.04 a | 5.05 ± 0.55 a | 5.07 ± 0.75 a | 2.95 ± 0.10 c | 3.17 ± 0.20 b | |
| BU Acc 32 | 17.1 ± 0.68 cd | 16.9 ± 0.89 d | 0.309 ± 0.03 ab | 0.304 ± 0.04 ab | 5.03 ± 0.50 a | 5.04 ± 0.78 a | 3.35 ± 0.20 abc | 3.24 ± 0.15 b | |
| BU Acc 37 | 18.5 ± 0.61 a | 18.1 ± 1.13 a | 0.295 ± 0.02 cd | 0.307 ± 0.04 ab | 5.11 ± 0.45 a | 5.09 ± 0.81 a | 3.74 ± 0.10 a | 3.61 ± 0.15 a | |
| BRRI dhan43 | 17.4 ± 0.55 bc | 17.8 ± 1.05 ab | 0.287 ± 0.03 d | 0.299 ± 0.05 bc | 5.06 ± 0.50 a | 5.03 ± 0.73 a | 3.55 ± 0.20 ab | 3.35 ± 0.20 ab | |
| Moisture stress |
BU Acc 5 | 6.2 ± 0.45 j | 6.4 ± 0.58 j | 0.139 ± 0.01 i | 0.140 ± 0.01 h | 1.29 ± 0.09 f | 1.31 ± 0.11 f | 1.13 ± 0.08 f | 1.15 ± 0.10 f |
| BU Acc 7 | 10.8 ± 0.74 h | 10.6 ± 0.83 i | 0.183 ± 0.01 h | 0.178 ± 0.01 g | 1.67 ± 0.10 de | 1.66 ± 0.21 de | 1.64 ± 0.08 e | 1.61 ± 0.13 e | |
| BU Acc 12 | 9.9 ± 0.71 i | 10.1 ± 1.06 i | 0.188 ± 0.01 h | 0.183 ± 0.02 g | 1.76 ± 0.10 cde | 1.78 ± 0.15 cde | 1.92 ± 0.07 de | 1.88 ± 0.15 de | |
| BU Acc 24 | 11.9 ± 0.65 g | 11.6 ± 1.07 h | 0.199 ± 0.02 g | 0.201 ± 0.02 ef | 1.96 ± 0.09 bcd | 1.95 ± 0.24 cd | 1.59 ± 0.07 ef | 1.56 ± 0.13 e | |
| BU Acc 25 | 12.7 ± 0.61 f | 12.8 ± 1.11 g | 0.217 ± 0.01 ef | 0.214 ± 0.03 de | 2.16 ± 0.08 bcd | 2.17 ± 0.31 bcd | 1.82 ± 0.07 de | 1.83 ± 0.10 de | |
| BU Acc 32 | 12.9 ± 0.75 f | 12.8 ± 1.13 g | 0.214 ± 0.02 ef | 0.213 ± 0.02 de | 2.31 ± 0.10 bc | 2.35 ± 0.25 bc | 2.25 ± 0.10 d | 2.15 ± 0.10 d | |
| BU Acc 37 | 14.9 ± 0.81 e | 14.8 ± 1.14 f | 0.218 ± 0.02 e | 0.221 ± 0.01 d | 2.42 ± 0.09 b | 2.46 ± 0.35 b | 3.03 ± 0.08 c | 2.94 ± 0.13 c | |
| BRRI dhan43 | 12.8 ± 0.70 f | 12.6 ± 1.36 g | 0.208 ± 0.02 fg | 0.211 ± 0.02 de | 2.31 ± 0.09 bc | 2.33 ± 0.30 bc | 2.17 ± 0.10 d | 2.07 ± 0.13 d | |
Significantly different mean values of individual column are shown in dissimilar letters based on Tukey’s test at 5 % level of significance. The data of five replicates ± SE are shown. SE: Standard error.
3.2.2. Stomatal conductance
Table 2 showed that deficit moisture significantly reduced the stomatal conductance (gs) of all the genotypes. However, under deficit moisture, genotype BU Acc 37 exposed the highest gs (1st year: 0.218 mmol m−2 s−1; 2nd year: 0.221 mmol m−2 s−1) followed by BU Acc 25, whereas genotype BU Acc 5 exposed the lowest gs (1st year: 0.139 mmol m−2 s−1; 2nd year: 0.140 mmol m−2 s−1). Significant variation was found in reduction of gs of different genotypes of rice, while genotype BU Acc 37 exhibited the lowest (1st year: 26.1 %; 2nd year: 28.0 %) next BU Acc 32, while genotype BU Acc 5 exhibited the highest (1st year: 55.2 %; 2nd year: 55.0 %).
3.2.3. Rate of transpiration
In this experiment, transpiration rate (Tr) of rice genotypes was decreased significantly due to deficit moisture (Table 2). But, genotype BU Acc 37 exposed the highest Tr (1st year: 2.42 mmol m−2 s−1; 2nd year:2.46 mmol m−2 s−1) next to BU Acc 32, whereas genotype BU Acc 5 showed the lowest Tr (1st year: 1.29 mmol m−2 s−1; 2nd year: 1.31 mmol m−2 s−1) under moisture stress. Tolerant genotypes showed higher stomatal conductance as well as higher Tr than the susceptible genotype under moisture stress. As a result, tolerant genotype like BU Acc 37 exposed the lowest rate of reduction (1st year: 52.6 %; 2nd year: 51.7 %) after that BU Acc 32, while susceptible genotype BU Acc 5 exposed the highest rate (1st year: 74.2 %, and 2nd year: 73.6 %).
3.3. Photosynthetic pigment
Table 2 shows the remarked reduction of total chlorophyll contents of different rice genotypes under deficit moisture. However, under deficit soil moisture, genotype BU Acc 37 exposed the maximum total chlorophyll content (1st year: 3.03 mg g−1; 2nd year: 2.94 mg g−1) after that BU Acc 32 (1st year: 2.25 mg g−1; 2nd year: 2.15 mg g−1), while BU Acc 5 exposed the minimum total chlorophyll content (1st year: 1.13 mg g−1; 2nd year: 1.15 mg g−1). Genotype BU Acc 37 also exposed the lowest rate of reduction (1st year: 18.9 %; 2nd year: 18.6 %) after that BU Acc 32 (1st year: 33.3 %; 2nd year: 33.6 %), whereas genotype BU Acc 5 exposed the highest rate of reduction (1st year: 64.5 %; 2nd year: 63.8 %).
3.4. Compatible solutes accumulation
3.4.1. Proline accumulation
Regardless of genotypes, proline content was markedly increased under deficit moisture (Table 3). Statistically the highest proline accumulation was found in genotype BU Acc 37 (1st year: 3.71 µg g−1 FW; 2nd year: 3.75 µg g−1 FW) after that BU Acc 32 (1st year: 3.52 µg g−1 FW; 2nd year: 3.51 µg g−1 FW), while genotype BU Acc 5 showed the lowest accumulation (1st year: 2.93 µg g−1 FW; 2nd year: 2.89 µg g−1 FW). Genotype BU Acc 37 also exposed the highest rate of increase (1st year: 145.7 %; 2nd year: 146.7 %) after that BU Acc 32 (1st year: 127.1 %; 2nd year: 132.5 %), whereas BU Acc 5 exposed the lowest rate of increase (1st year: 104.9 %; 2nd year: 95.3 %).
Table 3.
Effects of interaction between two moisture levels and different rice genotypes on osmoprotectants content of rice.
| Moisture levels | Genotypes | Content of proline (µg/ g FW) |
Content of soluble (mg/ g FW) |
||
|---|---|---|---|---|---|
| Year I | Year II | Year I | Year II | ||
| Control | BU Acc 5 | 1.43 ± 0.11 h | 1.48 ± 0.15 gh | 27.65 ± 1.15 cd | 27.81 ± 2.15 c |
| BU Acc 7 | 1.52 ± 0.11 gh | 1.55 ± 0.09 g | 27.11 ± 1.09 e | 27.55 ± 1.85 cd | |
| BU Acc 12 | 1.51 ± 0.12 gh | 1.52 ± 0.07 g | 27.61 ± 1.15 d | 27.60 ± 1.74 cd | |
| BU Acc 24 | 1.49 ± 0.11 gh | 1.48 ± 0.15 gh | 27.95 ± 1.20 c | 27.88 ± 1.68 c | |
| BU Acc 25 | 1.53 ± 0.10 g | 1.50 ± 0.15 g | 27.66 ± 1.15 cd | 27.75 ± 1.95 cd | |
| BU Acc 32 | 1.55 ± 0.10 g | 1.51 ± 0.10 g | 28.62 ± 1.11 a | 28.32 ± 2.08 a | |
| BU Acc 37 | 1.51 ± 0.08 gh | 1.52 ± 0.10 g | 28.81 ± 1.20 a | 28.55 ± 2.15 a | |
| BRRI dhan43 | 1.52 ± 0.08 gh | 1.50 ± 0.10 g | 28.28 ± 1.20 b | 28.15 ± 2.07 b | |
| Moisture stress |
BU Acc 5 | 2.93 ± 0.25 f | 2.89 ± 0.22 f | 16.33 ± 1.15 l | 16.51 ± 1.55 l |
| BU Acc 7 | 3.03 ± 0.22 e | 3.04 ± 0.15 e | 17.25 ± 1.20 k | 17.34 ± 1.65 k | |
| BU Acc 12 | 3.11 ± 0.22 de | 3.15 ± 0.25 d | 17.81 ± 1.15 j | 17.85 ± 1.55 j | |
| BU Acc 24 | 3.18 ± 0.20 d | 3.11 ± 0.25 d | 18.73 ± 1.15 i | 18.45 ± 1.50 i | |
| BU Acc 25 | 3.31 ± 0.25 c | 3.30 ± 0.30 c | 21.99 ± 1.25 h | 22.15 ± 1.32 h | |
| BU Acc 32 | 3.52 ± 0.25 b | 3.51 ± 0.25 b | 23.04 ± 1.22 g | 23.45 ± 1.55 f | |
| BU Acc 37 | 3.71 ± 0.15 a | 3.75 ± 0.20 a | 24.95 ± 1.25 f | 25.21 ± 1.30 e | |
| BRRI dhan43 | 3.38 ± 0.15 c | 3.35 ± 0.20 bc | 22.28 ± 1.20 h | 23.06 ± 1.28 g | |
Significantly different mean values of individual column are shown with dissimilar letters based on Tukey’s test at 5 % level of significance. The data of five replicates ± SE are shown. SE: Standard error.
3.4.2. Soluble sugar accumulation
Regardless of genotypes, soluble sugar accumulation was decreased significantly under deficit moisture (Table 3). However, under moisture stress, tolerant BU Acc 37 genotype exhibited the highest soluble sugar accumulation (1st year: 24.95 mg g−1 FW; 2nd year: 25.21 mg g−1 FW) after that BU Acc 32 (1st year: 23.04 mg g−1 FW; 2nd year: 23.45 mg g−1 FW), whereas susceptible BU Acc 5 genotype exhibited the lowest accumulation (1st year: 16.33 mg g−1 FW; 2nd year: 16.51 mg g−1 FW). The percent decrease over control was the lowest in tolerant genotype BU Acc 37 (1st year: 13.4 %; 2nd year: 11.7 %) after that BU Acc 32 (1st year: 19.5 %; 2nd year: 17.2 %), while susceptible BU Acc 5 genotype showed the highest percent decrease (1st year: 40.9 %; 2nd year: 40.6 %).
3.5. Accumulation of hydrogen peroxide and malondialdehyde
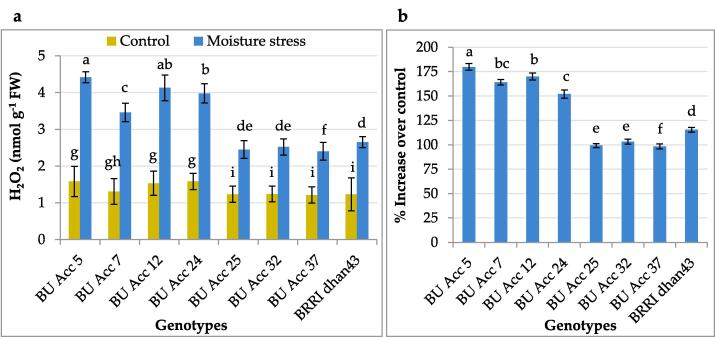
Fig. 1 shows that deficit soil moisture significantly increased the content of hydrogen peroxide (H2O2) of rice genotypes. However, under deficit moisture stress, susceptible BU Acc 5 genotype exposed the maximum accumulation of H2O2 (4.42 nmol g−1) after that BU Acc 12 (4.13 nmol g−1), while tolerant BU Acc 37 genotype exposed the minimum accumulation (2.40 nmol g−1) after that BU Acc 25 (2.45 nmol g−1). Susceptible BU Acc 5 genotype exposed the highest rate of increase (180 %) followed by BU Acc 12 (170 %), while tolerant BU Acc 37 genotype exposed the lowest rate of increase (98 %) followed by BU Acc 32 (99 %) (Fig. 1b).
Fig. 1.
Hydrogen peroxide (H2O2) content (a) and percent increase of H2O2 over control (b) in leaves of different rice genotypes with deficit moisture stress. Means of five replicates are shown through vertical bar. Standard error is shown by error bar. Significantly different mean values are shown in different letters (Tukey’s test at p ≤ 0.05).
Overproduction of malondialdehyde (MDA) under moisture stress is considered as the indicators of oxidative stress. Regardless of genotypes, deficit moisture markedly increased MDA accumulation (Fig. 2a). However, tolerant BU Acc 37 genotype exhibited the minimum MDA accumulation (30.9 nmol g−1) after that BU Acc 32 (32.6 nmol g−1), while susceptible BU Acc 5 genotype exhibited the maximum MDA accumulation (43.3 nmol g−1) after that BU Acc 7 (40.0 nmol g−1). Tolerant genotype BU Acc 37 also exhibited the lowest rate of increase (71 %) after that BU Acc 32 (87 %), whereas susceptible BU Acc 5 exhibited the highest rate of increase (135 %).
Fig. 2.
Melondealdehyde (MDA) content (a) and percent increase of MDA over control (b) in leaves of different rice genotypes with deficit moisture stress. Means of five replicates are shown through vertical bar. Standard error is shown by error bar. Significantly different mean values are shown in different letters (Tukey’s test at p ≤ 0.05).
3.6. Activity of antioxidant enzyme
Ascorbate peroxidase (APX) was markedly increased under deficit moisture in all rice genotypes (Fig. 3b). However, catalase (CAT) and superoxide dismutase (SOD) activity of all rice genotypes were markedly decreased under deficit moisture (Fig. 3a&c). Drought stress tolerance of rice genotypes also realizes by percent increase or percent decrease compare to control. Here, tolerant genotype BU Acc 37 exposed the maximum rate of APX activity increase (78.1 %) after that BU Acc 32 (66.5 %), while susceptible genotype BU Acc 5 exposed the minimum rate of increase (34.9 %). The lowest percent decrease of CAT and SOD activity was observed also in tolerant genotype BU Acc 37 (22.2 % and 14.5 % of CAT and SOD, respectively) followed by BU Acc 32 (26.3 % and 15.5 % of CAT and SOD, respectively), while the highest percent decrease was saw also in susceptible BU Acc 5 (44.8 % and 43.3 % of CAT and SOD, respectively).
Fig. 3.
Activities of different antioxidant enzyme of different rice genotypes under deficit moisture stress. (a) activity of catalase (CAT), (b) activity of ascorbate peroxidase (APX), and (c) activity of superoxide dismutase (SOD). Means of five replicates are shown through vertical bar. Standard error is shown by error bar. Significantly different mean values are shown in different letters (Tukey’s test at p ≤ 0.05).
3.7. Rice yield
3.7.1. Plant height
Deficit moisture markedly reduced the height of rice (Table 4). Under control condition, genotype BU Acc 25 exposed the maximum height (168.5 cm), while BRRI dhan43 exposed the minimum height (100.7 cm). Under deficit moisture, genotypes BU Acc 25 exposed the maximum height (143.7 cm), whereas BRRI dhan43 exposed the minimum (88.8 cm). However, tolerant BU Acc 37 genotypes showed the lowest reduction of plant height (8.7 %) followed by genotype BU Acc 32 (14.8 %), while susceptible genotypes BU Acc 5 showed the highest reduction (28.4 %). Other genotypes showed moderate plant height reduction.
Table 4.
Interaction effects of two moisture levels and different rice genotypes on yield attributes and yield of rice.
| Moisture levels | Genotypes | Height of plant (cm) | Total tillers/ hill | Effective tillers/ hill | Length of panicle (cm) | Filled grain per panicle | Weight of thousand- grain (g) | Grain yield (g/ hill) |
|---|---|---|---|---|---|---|---|---|
| Control | BU Acc 5 | 158.0 ± 7.3 b | 18.2 ± 1.06 ab | 16.2 ± 1.10 ab | 23.6 ± 1.45 b | 84.7 ± 5.5 e | 20.0 ± 1.10 ef | 25.8 ± 1.35 d |
| BU Acc 7 | 102.6 ± 5.6 f | 18.9 ± 1.25 a | 16.a ± 1.21 ab | 24.7 ± 1.32 b | 88.3 ± 5.8 d | 18.5 ± 1.08 h | 25.4 ± 1.26 d | |
| BU Acc 12 | 118.2 ± 5.2 de | 18.2 ± 1.34 ab | 15.9 ± 1.08 ab | 24.6 ± 1.55 b | 83.0 ± 4.4 ef | 20.1 ± 1.30 def | 23.2 ± 1.22 e | |
| BU Acc 24 | 167.7 ± 7.9 a | 19.2 ± 1.22 a | 17.1 ± 1.15 a | 24.0 ± 1.45 b | 82.3 ± 4.6 f | 21.7 ± 1.25 ab | 25.2 ± 1.15 d | |
| BU Acc 25 | 168.5 ± 8.3 a | 18.2 ± 1.24 ab | 15.9 ± 1.14 ab | 24.2 ± 1.25 b | 90.3 ± 3.5 c | 20.7 ± 1.25 cde | 27.8 ± 1.24 c | |
| BU Acc 32 | 142.0 ± 4.6 c | 18.5 ± 1.08 a | 16.1 ± 1.15 a | 23.1 ± 1.30 bc | 81.3 ± 2.8 f | 21.7 ± 1.14 ab | 26.8 ± 1.08 cd | |
| BU Acc 37 | 136.7 ± 5.2 c | 19.5 ± 1.22 a | 17.1 ± 1.10 a | 26.9 ± 1.22 a | 97.3 ± 2.7 a | 22.2 ± 1.11 a | 35.9 ± 2.11 a | |
| BRRI dhan43 | 100.7 ± 5.4 f | 18.4 ± 1.24 ab | 16.0 ± 1.24 ab | 23.7 ± 1.35 b | 95.7 ± 3.5 ab | 20.3 ± 1.25 def | 28.2 ± 1.24 c | |
| Moisture stress |
BU Acc 5 | 113.2 ± 6.8 e | 9.3 ± 1.06 g | 8.5 ± 0.91 f | 16.6 ± 1.25 e | 40.5 ± 2.6 j | 17.1 ± 1.06 i | 5.9 ± 0.8 i |
| BU Acc 7 | 86.3 ± 4.7 g | 13.7 ± 1.22 f | 12.5 ± 1.31 d | 20.4 ± 1.36 d | 78.3 ± 1.9 g | 17.1 ± 1.05 i | 16.8 ± 1.25 h | |
| BU Acc 12 | 103.7 ± 6.9 f | 13.3 ± 1.25 f | 11.5 ± 1.05 e | 20.2 ± 1.51 d | 73.7 ± 1.6 i | 19.1 ± 1.18 gh | 16.2 ± 1.15 h | |
| BU Acc 24 | 138.2 ± 8.2 c | 15.7 ± 1.25 de | 12.0 ± 1.15 de | 20.2 ± 1.45 d | 74.5 ± 2.4 hi | 20.9 ± 1.25 cd | 18.7 ± 1.15 g | |
| BU Acc 25 | 143.7 ± 7.8 c | 14.3 ± 1.18 ef | 12.9 ± 1.04 cd | 20.8 ± 1.50 d | 83.0 ± 1.5 ef | 19.8 ± 1.20 fg | 21.2 ± 1.20 f | |
| BU Acc 32 | 121.0 ± 7.3 de | 16.5 ± 1.31 bcd | 13.8 ± 1.10 c | 20.7 ± 1.50 d | 76.3 ± 1.06 h | 21.2 ± 1.17 bc | 22.2 ± 1.18 ef | |
| BU Acc 37 | 124.8 ± 8.3 d | 18.0 ± 1.27 abc | 15.6 ± 1.07 b | 25.1 ± 1.55 ab | 94.8 ± 1.8 b | 21.8 ± 1.35 ab | 31.1 ± 1.85 b | |
| BRRI dhan43 | 89.8 ± 5.3 g | 16.1 ± 1.11 cde | 12.7 ± 1.13 cd | 21.2 ± 1.40 cd | 88.3 ± 2.5 d | 19.7 ± 1.25 fg | 22.1 ± 1.25 ef | |
Significantly different mean values of individual column are shown with dissimilar letters based on Tukey’s test at 5 % level of significance. The data of five replicates ± SE are shown. SE: Standard error.
3.7.2. Total tillers per hill
Deficit moisture markedly reduced the total tillers number of rice genotypes (Table 4). At control condition, all rice genotypes showed statistically similar results. However, under deficit soil moisture, tolerant genotype BU Acc 37 exposed the maximum total tillers number (18.0) after that BU Acc 32 (16.5), while the minimum was detected in susceptible genotype BU Acc 5 (9.3). Tolerant genotypes BU Acc 37 also exposed the lowest rate of reduction of total tiller (7.8 %) followed by BU Acc 32 (11.0 %), while susceptible genotypes BU Acc 5 exposed the highest rate of reduction (48.9 %).
3.7.3. Effective tillers per hill
Deficit moisture markedly reduced the effective tillers number of rice genotypes (Table 4). However, under deficit moisture condition, the maximum number of effective tillers per hill was found in tolerant genotype BU Acc 37 (15.6) followed by BU Acc 32 (13.8), while genotype BU Acc 5 exposed the minimum (8.5). Tolerant genotypes BU Acc 37 also exposed the lowest reduction rate of effective tillers per hill (8.8 %) followed by BU Acc 32 (9.0 %), whereas susceptible genotypes BU Acc 5 exposed the highest reduction rate (44.0 %).
3.7.4. Panicle length
Deficit moisture stress significantly reduced the length of panicle of all rice genotypes (Table 4). Tolerant genotype BU Acc 37 exhibited the longest panicle (25.1 cm), while susceptible genotype BU Acc 5 exhibited the shortest panicle (16.6 cm) under deficit moisture. Tolerant genotypes BU Acc 37 exhibited the lowest rate of panicle reduction (6.7 %) followed by BU Acc 32 (10.4 %), while susceptible genotypes BU Acc 5 exhibited the highest rate of panicle reduction (29.7 %).
3.7.5. Filled grains per panicle
Filled grains per panicle of rice genotypes were markedly reduced by deficit moisture stress (Table 4). However, tolerant genotype BU Acc 37 exposed the maximum filled grains number per panicle (94.8), whereas susceptible genotype BU Acc 5 showed the minimum filled grains number per panicle (40.5) under deficit moisture. Tolerant genotypes BU Acc 37 also exposed the lowest rate of filled grain reduction per panicle (2.6 %) followed by BU Acc 32 (6.1 %), while susceptible genotypes BU Acc 5 showed the highest rate of reduction (52.2 %).
3.7.6. Thousand grain weight
Thousand grain weight of rice genotypes was significantly decreased under deficit moisture condition (Table 4). However, tolerant genotype BU Acc 37 exhibited the highest 1000-grain weight (21.8 g), after that BU Acc 32 (21.2 g), whereas susceptible genotype BU Acc 5 showed the lowest (17.07 g) under deficit moisture condition. Tolerant genotype BU Acc 37 also exhibited the lowest rate of 1000-grain weight reduction (1.88 %) after that BU Acc 32 (2.6 %), whereas susceptible genotype BU Acc 5 exhibited the highest rate of reduction (14.8 %).
3.7.7. Grain yield
Grain yield of different rice genotypes showed significant variation under deficit moisture condition. However, tolerant genotype BU Acc 37 showed the highest grain yield per hill (31.1 g) after that BU Acc 32 (22.2 g), while susceptible genotype BU Acc 5 showed the lowest grain yield per hill (5.9 g). Tolerant genotype BU Acc 37 exposed the lowest rate of grain yield reduction per hill (12.9 %) next BU Acc 32 (16.8 %) and the highest reduction percent of grain yield per hill was observed in susceptible genotype BU Acc 5 (77.2 %).
4. Discussion
Rice production is extremely squeezed due to scarcity of water. Plant hydrological state can be examine through different water relations characteristics of plant. In this study, water relations attributes of rice genotypes were markedly declined under deficit moisture condition (Table 1). Deficit moisture condition reduces the water absorption capacity of roots which reduces the water flow consequently hamper the water relations characteristics of rice. Impacts of moisture stress on water relation characteristics also regulated by intensity and period of deficit moisture condition and species of crop (Urmi et al., 2023). Among the water relations attributes, relative water content (RWC) is the most important attributes of crops because it represent the difference between water potential and turgor potential (Gupta et al., 2020). Leaves of deficit moisture stressed plants exhibit higher reductions in RWC and water potential. Table 1 showed that better water relations were observed in tolerant rice genotypes BU Acc 37 and BU Acc 32 rather than vulnerable BU Acc 5 genotypes. Urmi et al. (2023) also reported that sensitive rice genotypes show higher decrease in RWC than tolerant rice genotype under moisture stress. Tamanna et al. (2023) shows the well association of different water relation attributes with stress tolerance of different genotypes. Deficit moisture stress reduces the water retention capacity by occurring higher damage in cell structures of crops (Urmi et al., 2023, Imtiaz et al., 2020). Deficit moisture condition increases the water saturation deficit while decreases osmotic potential of crops (Zhang et al., 2015). Water uptake ability and rate of water loss of different genotypes are different that’s why significant variation was observed in water relations characteristics of different genotypes.
Table 2 showed remarkable variation of different gas exchange attributes under sufficient and deficit moisture condition, while genotypes BU Acc 37 and BU Acc 32 showed satisfactory results under moisture stress. Lower stomatal aperture under deficit moisture condition prevents the entry of carbon dioxide into leaves, enhances the formation of extra electrons like reactive oxygen species (ROS) and reduces photosynthesis (Farooq et al., 2009, Mishra et al., 2018). Moisture stress reduces the photosynthetic rate of crops by lowering the leaf number and size, inducing stomatal closure, reducing carboxylation enzymes activities, synthesis of ATP, damage of photosynthetic apparatus (Gupta et al., 2020, Zhu et al., 2020). Light capturing ability, photosynthetic pigments production, Rubisco activity and photosynthesis decrease under deficit moisture because of lower number and reduce size of leaf (Panda et al., 2021, Farooq et al., 2009). Diffusion of CO2 into leaves increases through higher stomatal conductance (Table 2) which enhance photosynthetic rates (Tamanna et al., 2023, Mishra et al., 2018). Osmotic imbalance like unavailability of fluid in cell membrane occurs under deficit soil moisture stress which enhances electrolyte leakage and reduces Pn, gs and Tr (Baroowa and Gogoi, 2016, Alzarqaa et al., 2014). Tolerant rice genotypes have higher water uptake and use ability by the mechanism of lower transpiration rate and showed better performance of Pn, gs and Tr than the susceptible genotype (Table 1, Table 2).
Chlorophyll is most widely used physiological indicators which directly regulates crop photosynthesis (Croft et al., 2017, Ashraf and Harris, 2013). Water scarcity gradually reduces the content of chlorophyll because drought stress hampers chlorophyll synthesis by damaging mesophyll chloroplasts (Abdelaal et al., 2020). Table 2 showed that chlorophyll contents of rice genotypes were decreased under deficit moisture. However, decreasing rate of chlorophyll contents was lower in tolerant genotypes than susceptible which also reflect on higher Pn, gs and Tr of tolerant genotypes. Urmi et al. (2023) found superior level of chlorophyll content consequently better performance in tolerant variety of rice under moisture stress condition. Moisture stress decreases the light harvest capacity and utilization of CO2 by mesophyll cells, consequently decrease chlorophyll content (Sarwar et al., 2013).
Numerous adaptive mechanisms are developed by plants to resolve the physiological damage under moisture stress (Panda et al., 2021, Urmi et al., 2023). Under moisture stress condition, accumulation of osmolytic cytosolutes is considered as stress tolerance indicator (Gao et al., 2020, Panda et al., 2021). Results of this study showed substantial variation among the rice genotypes regarding to proline as well as soluble sugar accumulation under deficit moisture. However, tolerant BU Acc 37 and BU Acc 32 genotypes showed significantly higher proline accumulation (Table 3). Therefore, genotypes BU Acc 37 and BU Acc 32 might be considered as drought tolerant promising genotype because proline is one of the most important osmolytic cytosolutes which mitigates moisture stress by osmotic regulation (Urmi et al., 2023). Proline stabilizes the mitochondrial electron transport system II, plays as electron receptor, scavenge ROS, increase antioxidant enzyme activity and protected plants against deficit moisture stress (Hare et al., 1998, Yaish, 2015, Saha et al., 2019). Soluble sugar content of different rice genotypes was markedly decreased under deficit moisture, while, minimum decreasing rate was observed in tolerant genotypes BU Acc 37 and BU Acc 32; and maximum was found in susceptible genotype BU Acc 5 (Table 3). Some studies show higher accumulation of soluble sugar under deficit moisture. Plants accumulate higher amount of soluble sugar for drought-induced damage repairing. Therefore, long time deficit moisture stress will affect the synthesis of sugar by destroying the structure of plants (Li et al., 2015, Gao et al., 2020). Sugar is converted to energy and carbon for survival of crops during extended deficit moisture condition (Siaut et al., 2011).
Scarcity of available water disrupts the mitochondrial and chloroplastic electron transport pathway and enhances overproduction of ROS (Melandri et al., 2020, Islam et al., 2020, Panda et al., 2021, Urmi et al., 2023). Overproduction of ROS enhances the accumulation of malondialdehyde (MDA) via lipid peroxidation (Islam et al., 2016, Islam et al., 2019). This research showed that, rice genotypes were significantly different from each other regarding the accumulation of H2O2 and MDA (Fig. 1, Fig. 2). Similar with these findings, Urmi et al. (2023) also observe that drought-sensitive rice cultivar accumulates significantly higher amounts of H2O2 and MDA than the tolerant cultivar. It is reported that less accumulation of H2O2 and MDA are the sign of higher tolerance against stresses (Panda et al., 2021, Urmi et al., 2023). Highly toxic radical H2O2 enhances cell death (Gill and Tuteja, 2010a). Overproduction of H2O2 and MDA cause oxidative damage to crops by denature the proteins, lipids and deoxyribonucleic acid (Gill and Tuteja, 2010b). Overproduction of H2O2 and MDA increase osmotic suffering and electrolyte leakage by reducing cell membrane integrity [(Table 1; Shukla et al. (2012)].
Plant possesses different defense systems such as enzymatic and non-enzymatic antioxidant systems to overcome different stress condition. These antioxidant enzyme systems scavenge ROS and mitigate oxidative stress (Melandri et al., 2020, Panda et al., 2021, Urmi et al., 2023). Findings of these research also similar to the findings of Melandri et al. (2020), Mishra and Panda, 2017, Gao et al., 2020 and Urmi et al. (2023) where, regardless of genotypes, deficit moisture stress significantly increased the APX activity, while significantly decreased the CAT and SOD activity (Fig. 3). Lower activity of CAT and SOD were observed in rice genotypes might be because of long period of drought. Many other researchers also found the increasing activity of CAT and SOD during short-period of drought, whereas lower activity of those enzyme under long period of drought (Gao et al., 2020, Urmi et al., 2023). Significantly different levels of enzymes activity were observed between tolerant and susceptible genotypes of this research. However, the highest percent increase of APX activity and the lowest percent decrease of CAT and SOD activity were observed in tolerant genotype BU Acc 37 followed by BU Acc 32 (Fig. 3). Merwad et al. (2018) reported that plant species, stress intensity and extent of unavailability of water influence the enzymes activity.
Table 4 showed that regardless of genotypes, deficit moisture stress significantly reduced the effective tiller number, panicle length and grains of each panicle, weight of thousand grains consequently yield. Imbalance of water potential gradient under deficit moisture stress prevents the absorption of sufficient amount of water. Nutrient uptake of plant also reduces due to less water absorption which prevents cell division as well as cell elongation and crop growth (Islam et al., 2018, Islam et al., 2021). Plant shows early maturity under moisture stress condition which badly affects the panicle length and grain per panicle as well as yield of crops (Sokoto and Muhammad, 2014). Scarcity of soil moisture at flowering stage is responsible for stigma drying. Lower germination of pollen grain in dry stigma reduces the formation of filled grain. Early maturity of different plant parts under deficit moisture stress decreases assimilates flow from lower to upper parts, causes ovules abortion which causes spikelet sterility, reduces filled grain per panicle consequently lower grain yield (Swain et al., 2017, Singh et al., 2017, Islam et al., 2021). Moisture stress enhances stomatal closure and reduces water and nutrient absorption, CO2 uptake which impairs photosynthesis and leads to lower grain yield (Du et al., 2015). Moreover, moisture stress decrease leaf area, chlorophyll content, hence reduces photosynthesis and grain yield [Table 2; Urmi et al. (2023)]. However, tolerant genotypes showed lower impact of moisture stress on different physiological attributes and ensure higher yield than the susceptible genotypes. These variation among the genotypes might be governed by various kind of genes which regulate their different characteristics (Eskandari and Kazemi, 2010, Panda et al., 2021, Urmi et al., 2023).
5. Conclusion
Drought stress sharply reduces the crop yield. Here, we studied the major physiological and morphological changes of rice under inadequate water condition. Deficit moisture condition extensively enhanced oxidative stress and lipid peroxidation which significantly decreased the major physiological attributes and yield of all rice genotypes. In contrast, among the genotypes, comparatively better physiological and morphological expressions were observed from genotypes BU Acc 37 and BU Acc 32 which might be due to higher oxidative stress tolerance of those genotypes through higher accumulation of osmolites and antioxidant enzyme activities. Therefore, genotypes BU Acc 37 and BU Acc 32 might be considered for future variety improvement.
Statement of author contributions
Khushi Rani Das; Farhana Zaman and Md. Moshiul Islam: Conceived and designed the experiments; conducted the experiments; analyzed and interpreted the data; wrote the paper.
Sazada Siddiqui, Mohammed O Alshaharni and Uthman Balgith Algopishi: Acquisition of fund; analyzed and interpreted the data; wrote the paper.
The authors extend their gratitude to the Deanship of Scientific Research at King Khalid University for funding this work through a Large Group Research Project under grant number RGP2/90/44. The authors also gratefully recognize the Research Management Wing, Bangabandhu Sheikh Mujibur Rahman Agricultural University, Gazipur, Bangladesh for partial funding and support of this experiment.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors extend their appreciation to the Deanship of Scientific Research at King Khalid University for funding this work through a Large Group Research Project under grant number RGP2/90/44. The authors also would like to extend their heartfelt gratitude to the Research Management Wing, Bangabandhu Sheikh Mujibur Rahman Agricultural University, Gazipur, Bangladesh.
Contributor Information
Khushi Rani Das, Email: khushi3481@stu.bsmrau.edu.bd.
Farhana Zaman, Email: farhana3541@stu.bsmrau.edu.bd.
Md. Moshiul Islam, Email: moshiul@bsmrau.edu.bd.
Sazada Siddiqui, Email: sasdeky@kku.edu.sa.
Mohammed O. Alshaharni, Email: maasalim@kku.edu.sa.
Uthman Balgith Algopishi, Email: ualgopishi@kku.edu.sa.
References
- Abdelaal K.A., Attia K.A., Alamery S.F., El-Afry M.M., Ghazy A.I., Tantawy D.S., Al-Doss A.A., El-Shawy E.S.E.M., Abu-Elsaoud A., Hafez Y.M. Exogenous application of proline and salicylic acid can mitigate the injurious impacts of drought stress on barley plants associated with physiological and histological characters. Sustainability. 2020;12:1736. [Google Scholar]
- Alzarqaa, A., Roushdy, S., Alderfasi, A., Al-Yahya, F., Dawabah, A., 2014. The physiological response of mungbean (Vigna radiata) to water deficit stress and Meloidogyne javanica infection. In Proceedings of the Sustainable Irrigation and Drainage V: Management, Technologies and Policies 185, 89–100.
- Anjum S.A., Xie X.Y., Wang L.C., Saleem M.F., Man C., Lei W. Morphological, physiological and biochemical responses of plants to drought stress. Afr. J. Agric. Res. 2011;6:2026–2032. [Google Scholar]
- Ashraf M., Harris P.J.C. Photosynthesis under stressful environments: An overview. Photosynthetica. 2013;51:163–190. [Google Scholar]
- Baroowa B., Gogoi N. Morpho-physiological and yield responses of Black gram (Vigna mungo L.) and Green gram (Vigna radiata L.) genotypes under drought at different growth stages. Res. J. Recent Sci. 2016;2277:2502. [Google Scholar]
- BRRI, Modern Rice Cultivation (Adunik Dhaner Chash), 2020, 23rd ed., Bangladesh Rice Research Institute: Gazipur, Bangladesh, 1–103.
- Croft H., Chen J.M., Luo X., Bartlett P., Chen B., Staebler R.M. Leaf chlorophyll content as a proxy for leaf photosynthetic capacity. Glob. Chang. Biol. 2017;23:3513–3524. doi: 10.1111/gcb.13599. [DOI] [PubMed] [Google Scholar]
- Dien D.C., Mochizuki T., Yamakawa T. Effect of various drought stresses and subsequent recovery on proline, total soluble sugar and starch metabolisms in Rice (Oryza sativa L.) varieties. Plant Prod. Sci. 2019;22:530–545. [Google Scholar]
- Du T., Kang S., Zhang J., Davies W.J. Deficit irrigation and sustainable water resource strategies in agriculture for China’s food security. J. Exp. Bot. 2015;66:2253–2269. doi: 10.1093/jxb/erv034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskandari H., Kazemi K.K. Response of different bread wheat (Triticum aestivum L.) genotypes to post-anthesis water deficit. Not. Sci. Biol. 2010;2:49–52. [Google Scholar]
- Fahad S.C., Bajwa A.A., Nazir U., Anjum S.A., Farooq A., Zohaib A., Sadia S., Nasim W., Adkins S., Saud S., et al. Crop production under drought and heat stress: plant responses and management options. Front. Plant Sci. 2017;8:1147. doi: 10.3389/fpls.2017.01147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farooq M., Kobayashi N., Wahid A., Ito O., Basra S.M.A. Strategies for producing more rice with less water. Adv. Agron. 2009;101:351–388. [Google Scholar]
- Gao S., Wang Y., Yu S., Huang Y., Liu H., Chen W., He X. Effects of drought stress on growth, physiology and secondary metabolites of Two Adonis species in Northeast China. Sci. Hortic. 2020;259 [Google Scholar]
- Gill S.S., Tuteja N. Polyamines and abiotic stress tolerance in plants. Plant Signal. Behav. 2010;5:26–33. doi: 10.4161/psb.5.1.10291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill S.S., Tuteja N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010;48:909–930. doi: 10.1016/j.plaphy.2010.08.016. [DOI] [PubMed] [Google Scholar]
- Gupta A., Rico-Medina A., Caño-Delgado A.I. The physiology of plant responses to drought. Science. 2020;368:266–269. doi: 10.1126/science.aaz7614. [DOI] [PubMed] [Google Scholar]
- Hare P.D., Cress W.A., Van Staden J. Dissecting the roles of osmolyte accumulation during stress. Plant Cell Environ. 1998:21535–22153. [Google Scholar]
- Hussain H.A., Hussain S., Khaliq A., Ashraf U., Anjum S.A., Men S., Wang L. Chilling and drought stresses in crop plants: Implications, cross talk, and potential management opportunities. Front. Plant Sci. 2018;9393 doi: 10.3389/fpls.2018.00393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imtiaz A.A., Shahriar S.A., Baque M.A., Eaty M.N.K., Falguni M.R. Screening of Mungbean Genotypes under Polyethylene Glycol (PEG) Induced Drought Stress Condition. Annu. Res. Rev. Biol. 2020;35:1–12. [Google Scholar]
- Islam M.M., Ye W., Matsushima D., Munemasa S., Okuma E., Nakamura Y., Biswas S., Mano J.I., Murata Y. Reactive carbonyl species mediate ABA signaling in guard cells. Plant Cell Physiol. 2016;57:2552–2563. doi: 10.1093/pcp/pcw166. [DOI] [PubMed] [Google Scholar]
- Islam M.M., Kayesh E., Zaman E., Urmi T.A., Haque M.M. Evaluation of rice (Oryza sativa L.) genotypes for drought tolerance at germination and early seedling stage. Agriculturists. 2018;16:44–54. [Google Scholar]
- Islam M.M., Ye W., Matsushima D., Rhaman M.S., Munemasa S., Okuma E., Nakamura Y., Biswas M.S., Mano J.I., Murata Y. Reactive carbonyl species function as signal mediators downstream of H2O2 production and regulate [Ca2+]cyt elevation in ABA signal pathway in Arabidopsis guard cells. Plant Cell Physiol. 2019;60:1146–1159. doi: 10.1093/pcp/pcz031. [DOI] [PubMed] [Google Scholar]
- Islam M.M., Ye W., Akter F., Rhaman M.S., Matsushima D., Munemasa S., Okuma E., Nakamura Y., Biswas M.S., Mano J.I., et al. Reactive carbonyl species mediate methyl jasmonate-induced stomatal closure. Plant Cell Physiol. 2020;61:1788–1797. doi: 10.1093/pcp/pcaa107. [DOI] [PubMed] [Google Scholar]
- Islam M.M., Ahmed S., Urmi T.A., Raihan M.S., Islam M.R. Evaluation of Moisture Regime on Agronomic Traits of Rice Genotypes. Ann. Bangladesh Agric. 2021;25:89–104. [Google Scholar]
- Islam M.M., Jahan K., Sen A., Urmi T.A., Haque M.M., Ali H.M., Siddiqui M.H., Murata Y. Exogenous application of calcium ameliorates salinity stress tolerance of tomato (Solanum lycopersicum L.) and enhances fruit quality. Antioxidants. 2023;12:558. doi: 10.3390/antiox12030558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurepin LV, Ivanov AG, Zaman M, Pharis RP, Hurry V, Hüner NP., 2017. Interaction of glycine betaine and plant hormones: Protection of the photosynthetic apparatus during abiotic stress. Photosynthesis: Structures, mechanisms, and applications, Springer International Publishing, Cham. 185-202.
- Li L., Liu Y., Liu Y., He B., Wang M., Yu C., Weng M. Physiological response and resistance of three cultivars of Acer rubrum L. to continuous drought stress. Acta Ecol. Sin. 2015;35:196–202. [Google Scholar]
- Li Z., Peng Y., Huang B. Physiological effects of γ-aminobutyric acid application on improving heat and drought tolerance in creeping bentgrass. J. Am. Soc. Hortic. Sci. 2016;141(1):76–84. [Google Scholar]
- Melandri G., AbdElgawad H., Riewe D., Hageman J.A., Asard H., Beemster G.T., Kadam N., Jagadish K., Altmann T., Ruyter-Spira C., et al. Biomarkers for grain yield stability in rice under drought stress. J. Exp. Bot. 2020;71:669–683. doi: 10.1093/jxb/erz221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merwad A.R.M., Desoky E.S.M., Rady M.M. Response of water deficit-stressed Vigna unguiculata performances to silicon, proline or methionine foliar application. Sci. Hortic. 2018;228:132–144. [Google Scholar]
- Mishra S.S., Behera P.K., Kumar V., Lenka S.K., Panda D. Physiological characterization and allelic diversity of selected drought tolerant traditional rice (Oryza sativa L.) landraces of Koraput. India. Physiol. Mol. Biol. Plants. 2018;24:1035–1046. doi: 10.1007/s12298-018-0606-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra S.S., Panda D. Leaf traits and antioxidant defense for drought tolerance during early growth stage in some popular traditional rice landraces from Koraput. India. Rice Sci. 2017;24:207–217. [Google Scholar]
- Molotoks A., Smith P., Dawson T.P. Impacts of land use, population, and climate change on global food security. Food Energy Secur. 2021;10:261. [Google Scholar]
- Noctor G., Reichheld J.P., Foyer C.H. ROS-related redox regulation and signaling in plants. Semin. Cell Dev. Biol. 2018;80:3–12. doi: 10.1016/j.semcdb.2017.07.013. [DOI] [PubMed] [Google Scholar]
- Panda D., Mishra S.S., Behera P.K. Drought tolerance in rice: Focus on recent mechanisms and approaches. Rice Sci. 2021;28:119–132. [Google Scholar]
- Rahdari P., Hosseini S.M., Tavakoli S. The studying effect of drought stress on germination, proline, sugar, lipid, protein and chlorophyll content in purslane (Portulaca oleracea L.) leaves. J. Med. Plant Res. 2012;6:1539–1547. [Google Scholar]
- Saha S., Begum H.H., Nasrin S. Effects of drought stress on growth and accumulation of proline in five rice varieties (Oryza sativa L.) J. Asiatic Soc. Bangladesh, Sci. 2019;45:241–247. [Google Scholar]
- Sarwar J.M., Nozulaidi B.N.M., Khairi B.C.L.M., Mohd K.Y. Effects of water stress on rice production: Bioavailability of potassium in soil. J. Stress Physiol. Biochem. 2013;9:97–107. [Google Scholar]
- Shelley I.J., Takahashi-Nosaka M., Kano-Nakata M., Haque M.S., Inukai Y. Rice cultivation in bangladesh: present scenario, problems, and prospects. JICAD. 2016;14:20–29. [Google Scholar]
- Shukla N., Awasthi R.P., Rawat L., Kumar J. Biochemical and physiological responses of rice (Oryza sativa L.) as influenced by Trichoderma harzianum under drought stress. Plant Physiol. Biochem. 2012;54:78–88. doi: 10.1016/j.plaphy.2012.02.001. [DOI] [PubMed] [Google Scholar]
- Siaut M., Cuiné S., Cagnon C., Fessler B., Nguyen M., Carrier P., Beyly A., Beisson F., Triantaphylidès C., Li-Beisson Y., et al. Oil accumulation in the model green alga Chlamydomonas reinhardtii: Characterization, variability between common laboratory strains and relationship with starch reserves. BMC Biotechnol. 2011;11:7. doi: 10.1186/1472-6750-11-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqui M.H., Alamri S., Al-Khaishany M.Y., Khan M.N., Al-Amri A., Ali H.M., Alaraidh I.A., Alsahli A.A. Exogenous melatonin counteracts NaCl-induced damage by regulating the antioxidant system, proline and carbohydrates metabolism in tomato seedlings. Int. J. Mol. Sci. 2019;20:353. doi: 10.3390/ijms20020353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh B., Reddy K.R., Redoña E.D., Walker T. Screening of rice cultivars for morpho-physiological responses to early-season soil moisture stress. Rice Sci. 2017;24:322–335. [Google Scholar]
- Sokoto M.B., Muhammad A. Response of rice varieties to water stress in Sokoto, Sudan Savannah. Nigeria. J. Biosci. Med. 2014;2:68–74. [Google Scholar]
- Souza T.C., de Castro E.M., Magalhaes P.C., Lino L.D.O., Alves E.T., de Albuquerque P.E.P. Morphophysiology, morphoanatomy, and grain yield under field conditions for two maize hybrids with contrasting response to drought stress. Acta Physiol. Plant. 2013;35(11):3201–3211. [Google Scholar]
- Swain P., Raman A., Singh S.P., Kumar A. Breeding drought tolerant rice for shallow rainfed ecosystem of eastern India. Field Crop. Res. 2017;209:168–178. doi: 10.1016/j.fcr.2017.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamanna T., Islam M.M., Chaity A.R., Shams S.N.U., Rasel M.A., Haque M.M., Miah M.G., Alamri S., Murata Y. Water Relation, Gas Exchange Characteristics and Yield Performance of Selected Mungbean Genotypes under Low Soil Moisture Condition. Agronomy. 2023;13:1068. [Google Scholar]
- Urmi T.A., Islam M.M., Zumur K.N., Abedin M.A., Haque M.M., Siddiqui M.H., Murata Y., Hoque M.A. Combined effect of salicylic acid and proline mitigates drought stress in rice (Oryza sativa L.) through the modulation of physiological attributes and antioxidant enzymes. Antioxidants. 2023;12(7):1438. doi: 10.3390/antiox12071438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaish M.W. Proline accumulation is a general response to abiotic stress in the date palm tree (Phoenix dactylifera L.) Genet. Mol. Res. 2015;14:9943–9950. doi: 10.4238/2015.August.19.30. [DOI] [PubMed] [Google Scholar]
- Yang X., Wang B., Chen L., Li P., Cao C. The different influences of drought stress at the flowering stage on rice physiological traits, grain yield, and quality. Sci. Rep. 2019;9(1):1–12. doi: 10.1038/s41598-019-40161-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M., Jin Z.Q., Zhao J., Zhang G., Wu F. Physiological and biochemical responses to drought stress in cultivated and Tibetan wild barley. Plant Growth Regul. 2015;75:567–574. [Google Scholar]
- Zhu R., Wu F.Y., Zhou S., Hu T., Huang J., Gao Y. Cumulative effects of drought-flood abrupt alternation on the photosynthetic characteristics of rice. Environ. Exp. Bot. 2020;169 [Google Scholar]