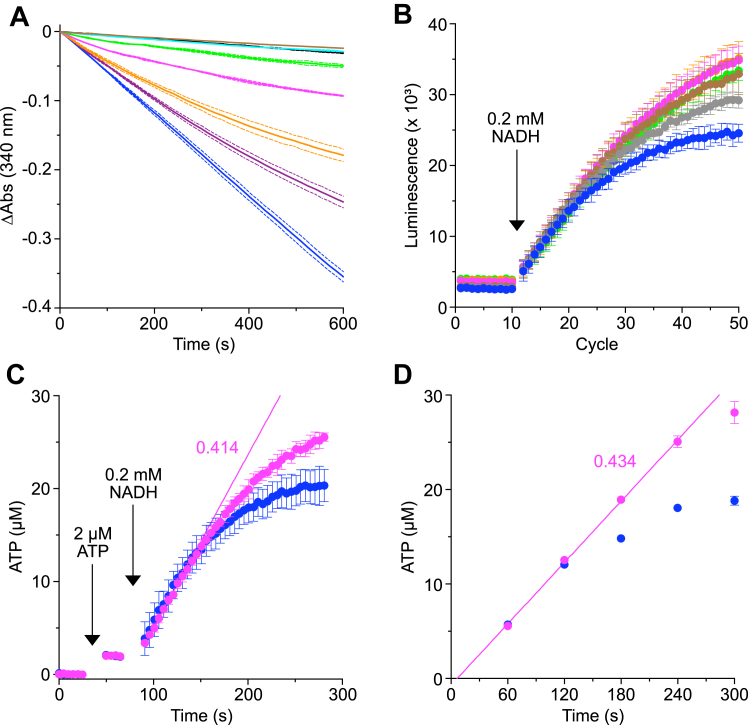
Figure 4.
Impact of constitutively active human IF1on ATP hydrolysis and ATP synthesis by bovine SMPs.A, ATP hydrolysis inhibited by increasing concentrations of constitutively active human IF1(1–81)-H49K. ( ), no inhibitor; (—), plus 1 μM oligomycin. IF1 at various concentrations; (
), no inhibitor; (—), plus 1 μM oligomycin. IF1 at various concentrations; ( ), 0.05 μM; (
), 0.05 μM; ( ), 0.1 μM; (
), 0.1 μM; ( ), 0.2 μM; (
), 0.2 μM; ( ), 0.4 μM; (
), 0.4 μM; ( ), 0.8 μM; (
), 0.8 μM; ( ), 1.6 μM. Measurements were made in triplicate, and the traces represent the average values corrected to an initial value of zero, and ± SD shown with bounding dashed lines (File S15). B–D, ATP synthesis by bovine SMPs coupled to NADH oxidation, in the presence or absence of human IF1(1–81)-H49K. ATP synthesis was initiated by the addition of NADH (0.2 mM). In (B) and (C), ATP synthesis was measured with a luminescence continuous real-time assay, and in (D), by a quench assay with luciferase-luciferin reagent. In (B), the concentrations of human IF1(1–81)-H49K were as follows: (
), 1.6 μM. Measurements were made in triplicate, and the traces represent the average values corrected to an initial value of zero, and ± SD shown with bounding dashed lines (File S15). B–D, ATP synthesis by bovine SMPs coupled to NADH oxidation, in the presence or absence of human IF1(1–81)-H49K. ATP synthesis was initiated by the addition of NADH (0.2 mM). In (B) and (C), ATP synthesis was measured with a luminescence continuous real-time assay, and in (D), by a quench assay with luciferase-luciferin reagent. In (B), the concentrations of human IF1(1–81)-H49K were as follows: ( ), no inhibitor; (
), no inhibitor; ( ), 0.2 μM; (
), 0.2 μM; ( ), 1 μM; (
), 1 μM; ( ), 5 μM; (
), 5 μM; ( ), 10 μM; (
), 10 μM; ( ), 20 μM. A concentration of 20 μM IF1 provides a molar excess of ca. 1600:1 with respect to the quantity of ATP synthase. Background luminescence levels were established for ten measurement cycles. N = 4 wells, and data points correspond to the average signal ± SD (File S16). In (C), the luminescence signal was calibrated with 2 μM ATP before the addition of NADH, and the background signal before addition of ATP was subtracted. (
), 20 μM. A concentration of 20 μM IF1 provides a molar excess of ca. 1600:1 with respect to the quantity of ATP synthase. Background luminescence levels were established for ten measurement cycles. N = 4 wells, and data points correspond to the average signal ± SD (File S16). In (C), the luminescence signal was calibrated with 2 μM ATP before the addition of NADH, and the background signal before addition of ATP was subtracted. ( ), no inhibitor; (
), no inhibitor; ( ), 5 μM IF1(1–81)-H49K. The data points are the average signal ± SD, n = 4 wells (File S17). D, ATP synthesis determined with the quench assay. (
), 5 μM IF1(1–81)-H49K. The data points are the average signal ± SD, n = 4 wells (File S17). D, ATP synthesis determined with the quench assay. ( ), no inhibitor; (
), no inhibitor; ( ), 5 μM IF1(1–81)-H49K, corresponding to an IF1:ATP synthase molar ratio of ca. 700:1. Data points are the average signal ± SD, n = 3 (File S18). In (C), a linear regression (
), 5 μM IF1(1–81)-H49K, corresponding to an IF1:ATP synthase molar ratio of ca. 700:1. Data points are the average signal ± SD, n = 3 (File S18). In (C), a linear regression ( ) was applied to the initial rate (25 s) of ATP synthesis in the presence of IF1 H49K, and in (D) to the rate over 240 s. The rates of ATP synthesis (magenta) in μmol min−1 mg−1 were calculated from the slopes. SMP, submitochondrial particle.
) was applied to the initial rate (25 s) of ATP synthesis in the presence of IF1 H49K, and in (D) to the rate over 240 s. The rates of ATP synthesis (magenta) in μmol min−1 mg−1 were calculated from the slopes. SMP, submitochondrial particle.