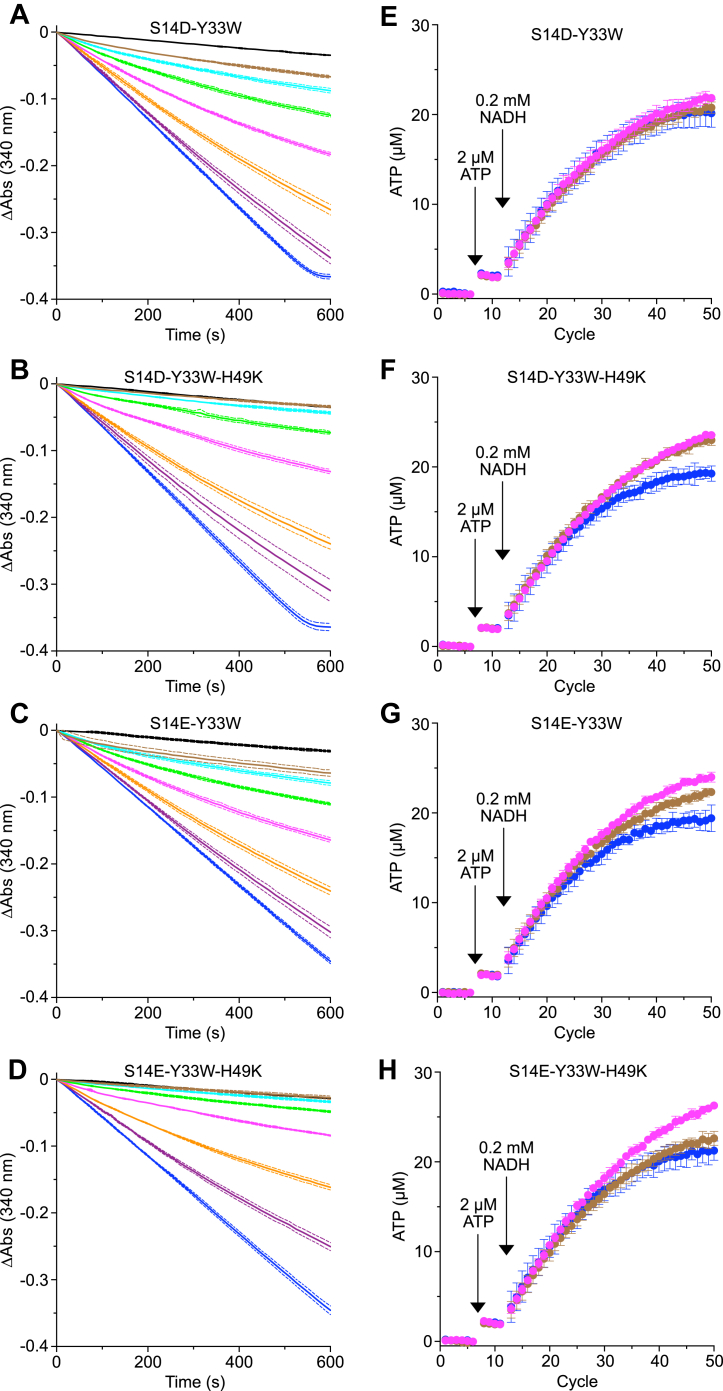
Figure 5.
Impact of phosphomimetic mutant forms of human IF1on the hydrolytic and synthetic activities of bovine ATP synthase.A–D, the impacts on ATP hydrolysis. E–H, the impacts on ATP synthesis. The following inhibitors were employed. In (A) and (E), IF1(1–81)-S14D-Y33W; in (B) and (F), IF1(1–81)-S14D-Y33W-H49K; in (C) and (G), IF1(1–81)-S14E-Y33W; in (D) and (H), IF1(1–81)-S14E-Y33W-H49K. In (A–D), inhibition of ATP hydrolysis by bovine SMPs by increasing concentrations of IF1. ( ), 0.05 μM; (
), 0.05 μM; ( ), 0.1 μM; (
), 0.1 μM; ( ), 0.2 μM; (
), 0.2 μM; ( ), 0.4 μM; (
), 0.4 μM; ( ), 0.8 μM; (
), 0.8 μM; ( ), 1.6 μM. (
), 1.6 μM. ( ) and (—), controls with no inhibitor and 1 μM oligomycin, respectively. Measurements were made in triplicate, and traces correspond to the average values corrected to an initial value of zero, with ± SD shown as bounding dashed lines (Files S19–S22). In (E–H), ATP synthesis by bovine heart SMPs coupled to NADH oxidation, measured by a luminescence continuous real-time assay and with a luciferase-luciferin reagent. The luminescence signal was calibrated with 2 μM ATP, and the background signal before addition of ATP was subtracted. ATP synthesis was initiated by the addition of NADH (0.2 mM). (
) and (—), controls with no inhibitor and 1 μM oligomycin, respectively. Measurements were made in triplicate, and traces correspond to the average values corrected to an initial value of zero, with ± SD shown as bounding dashed lines (Files S19–S22). In (E–H), ATP synthesis by bovine heart SMPs coupled to NADH oxidation, measured by a luminescence continuous real-time assay and with a luciferase-luciferin reagent. The luminescence signal was calibrated with 2 μM ATP, and the background signal before addition of ATP was subtracted. ATP synthesis was initiated by the addition of NADH (0.2 mM). ( ), no inhibitor; (
), no inhibitor; ( ), 0.5 μM IF1; (
), 0.5 μM IF1; ( ), 5 μM IF1. A concentration of 5 μM IF1 provides a molar ratio of ca. 400:1 with respect to the ATP synthase. Data points are the average signal ± SD, n = 4 wells (Files S23–S26). SMP, submitochondrial particle.
), 5 μM IF1. A concentration of 5 μM IF1 provides a molar ratio of ca. 400:1 with respect to the ATP synthase. Data points are the average signal ± SD, n = 4 wells (Files S23–S26). SMP, submitochondrial particle.