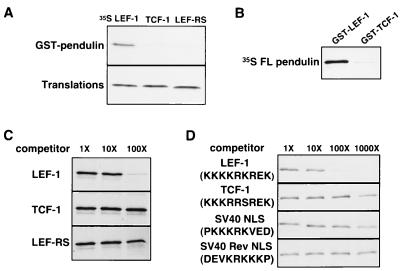
FIG. 2.
Specificity of an in vitro GST pull-down binding assay. (A) Sequences encoding the HMG DNA binding domains of LEF-1 (LEF (aa 297 to 384), TCF-1 (aa 152 to 239 of TCF-1A), and LEF-RS were in vitro translated and incubated with GST-pendulin fusion protein. 35S-labeled proteins that bound to the GST fusion proteins were sedimented with glutathione resin followed by SDS-PAGE of released proteins and autoradiography of the gel (GST pull-down assay described in the text and in Materials and Methods). Only the LEF-1 HMG DNA binding domain can bind to pendulin, and the 2-aa mutation (LEF-RS) destroys this interaction. (B) FL pendulin was generated by in vitro translation in reticulocyte lysates supplemented with [35S]methionine and then incubated with GST–LEF-1 or GST–TCF-1 in the GST pull-down assay. SDS-PAGE analysis shows that pendulin interacts with GST–LEF-1 but not GST–TCF-1. (C) The specificity of binding of in vitro-translated FL pendulin to GST–LEF-1 in the GST pull-down assay was analyzed by competition with 1-, 10-, and 100-fold molar excesses of purified, recombinant LEF-1, TCF-1, and LEF-RS HMG DNA binding domain proteins compared to the amount of GST-LEF-1 added. (D) As in panel C, the specificity of binding of in vitro-translated FL pendulin to GST–LEF-1 in the GST pull-down assay was analyzed by competition with 1-, 10-, 100-, and 1,000-fold molar excesses of peptides encoding the B boxes of LEF-1 and TCF-1, the SV40 T Ag NLS, and a mutant SV40 T Ag NLS which is the reverse sequence.