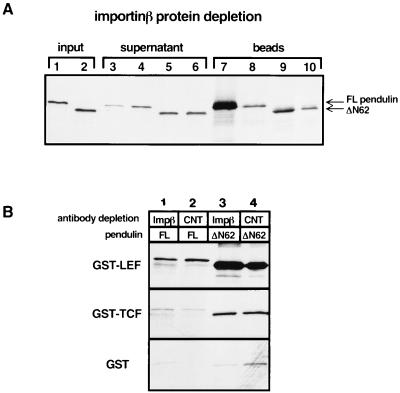
FIG. 6.
Importin-β does not influence the affinity or specificity of the pendulin–LEF-1 interaction. (A) Reticulocyte lysates programmed with constructs encoding either FL pendulin (lanes 1, 3, 4, 7, and 8) or ΔN62 pendulin (lanes 2, 5, 6, 9, and 10) were treated with importin-β antiserum (lanes 3, 5, 7, and 9) or control antiserum (lanes 4, 6, 8, and 10) covalently attached to beads. Lanes 1 and 2 depict 1.25% of the translation product in lysates prior to treatment with the antibody-coupled beads; lanes 3 through 6 show 1.25% of the translation product remaining after the antibody depletion; lanes 7 through 10 show 100% of the translation product that cosedimented with the antibody-coupled beads. (B) Analysis of the depleted lysates in the GST pull-down assay. Translation lysates treated with importin-β antisera (Impβ) or a control serum (CNT) were incubated with GST–LEF-1, GST–TCF-1, or GST alone in the GST pull-down assay. Depletion of importin-β from the reticulocyte lysates does not significantly affect the binding of pendulin to GST–LEF-1 or GST–TCF-1.