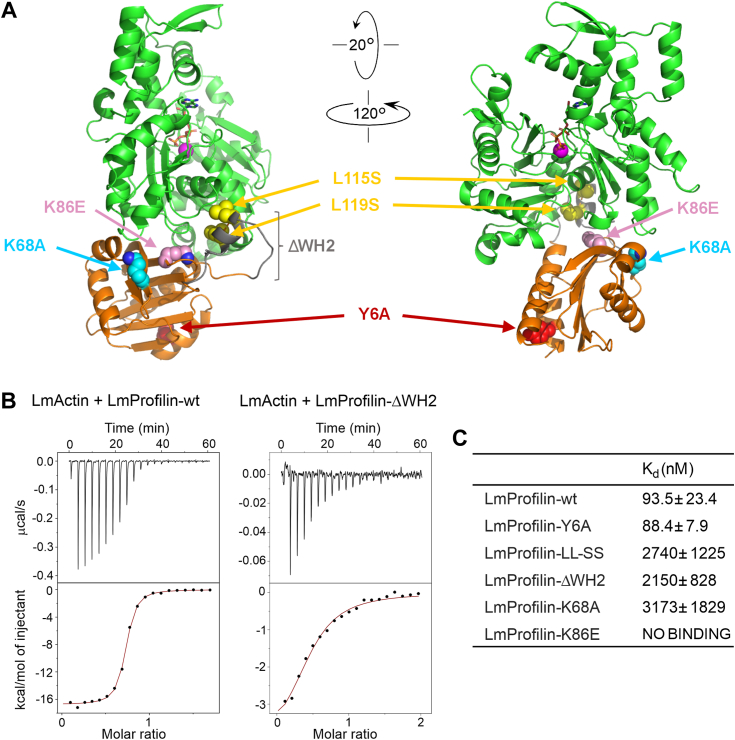
Figure 3.
Site-directed mutagenesis reveals the roles of different protein motifs of Leishmania profilin in actin binding.A, the locations of amino acid residues that were mutated in Leishmania profilin (yellow, L115 and L119; red, Y6A; gray, deleted WH2 motif; pink, K86; and cyan, K68) are indicated in the profilin–actin complex (shown in two different orientations). B, examples of the data from the isothermal titration calorimetry assay. Baseline-corrected thermograms (upper graphs) and integrated data fit to one-site binding model (lower graphs) are shown. C, dissociation constants (Kd, in nM ± SD) of WT and mutant Leishmania profilins from Leishmania ATP–actin monomers, obtained from three independent ITC experiments for each protein. ITC, isothermal titration calorimetry.