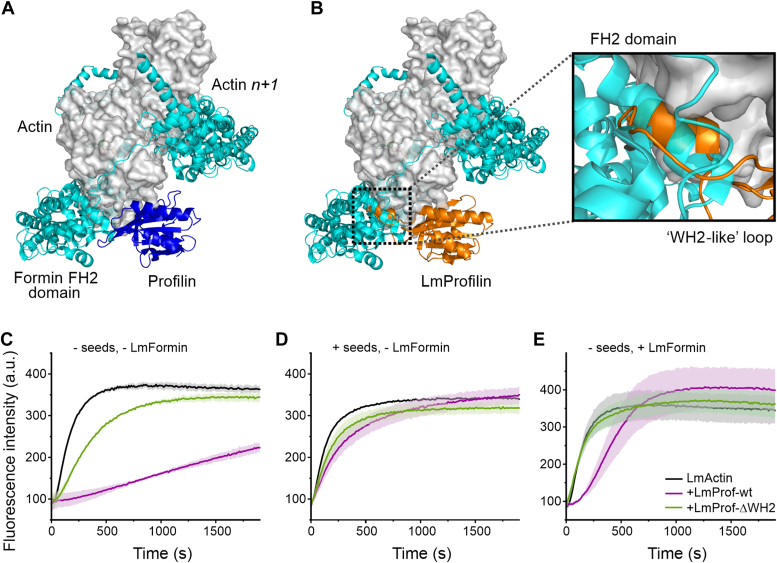
Figure 5.
Effects of Leishmania profilin on formin-catalyzed actin filament assembly.A, human profilin-I (Protein Data Bank ID: 2BTF) can be fitted to the barbed end of the “terminal” actin subunit of the 2:2 FH2 domain:actin monomer structure (Protein Data Bank ID: 1Y64) without steric clashes. B, superimposition of the Leishmania profilin from our cocrystal structure to the FH2:actin structure results in major steric clashes between the profilin WH2-like motif and the FH2 domain. C–E, pyrene–actin polymerization assays to monitor the effects of WT (LmProf_WT; purple curve) and LmProfilin-ΔWH2 (LmProf_ΔWH2; green curve) on spontaneous actin filament assembly (C), on actin filament assembly from actin–phalloidin seeds (D), and on actin filament assembly induced by LmFormin FH1–FH2 fragment (E). Final concentrations of actin (95% LmActin, 5% rabbit-pyrene actin) and profilin were 3 μM, and the concentrations of LmFormin FH1–FH2 and phalloidin seeds were 0.05 μM and 0.03 μM, respectively. Each curve depicts the average of four independent experiments with SD shown in lighter color. FH2, formin homology 2; LmActin, Leishmania major actin; LmFormin, L. major formin-B; WH2, WASP homology-2 domain.