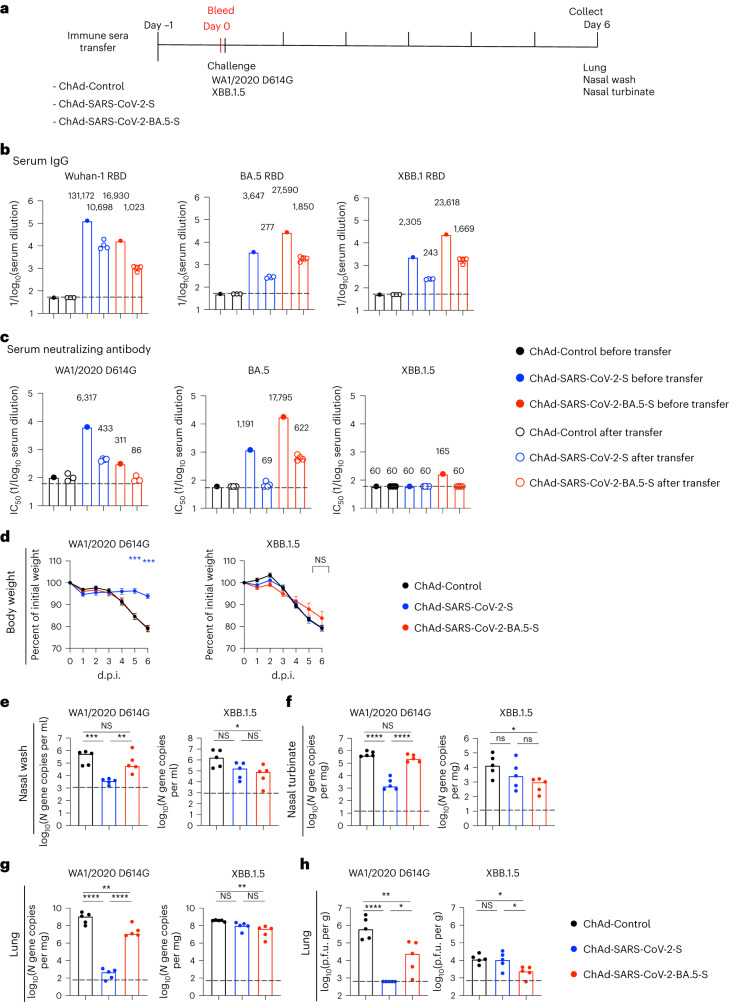
Fig. 7. Vaccine-induced serum antibody responses are insufficient to protect against XBB.1.5.
a, Scheme of serum transfer, blood collection and virus challenge. Seven- to 8-week-old female K18-hACE2 mice were administered pooled sera collected from mice immunized with ChAd-Control, ChAd-SARS-CoV-2-S or ChAd-SARS-CoV-2-BA.5-S vaccine (as described in Fig. 1b) by i.p. injection. b,c, Blood was collected from a subset of animals 24 h later, and serum antibody levels were determined. b, RBD-specific IgG to Wuhan-1, BA.5 and XBB.1. c, Serum neutralizing antibody titers against the indicated authentic SARS-CoV-2 strains (ChAd-Control, n = 3; ChAd-SARS-CoV-2-S, n = 4; ChAd-SARS-CoV-2-BA.5-S, n = 4). Boxes illustrate geometric mean values, numbers on top of the bar graph represent geometric mean values, and dotted lines show the LOD. d–h, One day after serum transfer, mice were challenged with 104 f.f.u. of WA1/2020 D614G or XBB.1.5 i.n. d, Body weight measurements (percent of initial weight) (mean ± s.e.m.; n = 5 mice per group). e–g, Levels of viral RNA in nasal washes (e), nasal turbinates (f) and lungs (g). h, Infectious virus in the lungs at 6 d.p.i. were analyzed (n = 5 mice per group). Boxes indicate median values, and dotted lines show the LOD. Data were analyzed by two-way ANOVA with a Tukey’s post hoc test (d) or one-way ANOVA with a Tukey’s post hoc test (e–h); *P < 0.05, **P < 0.01, ***P < 0.001 and ****P < 0.0001.