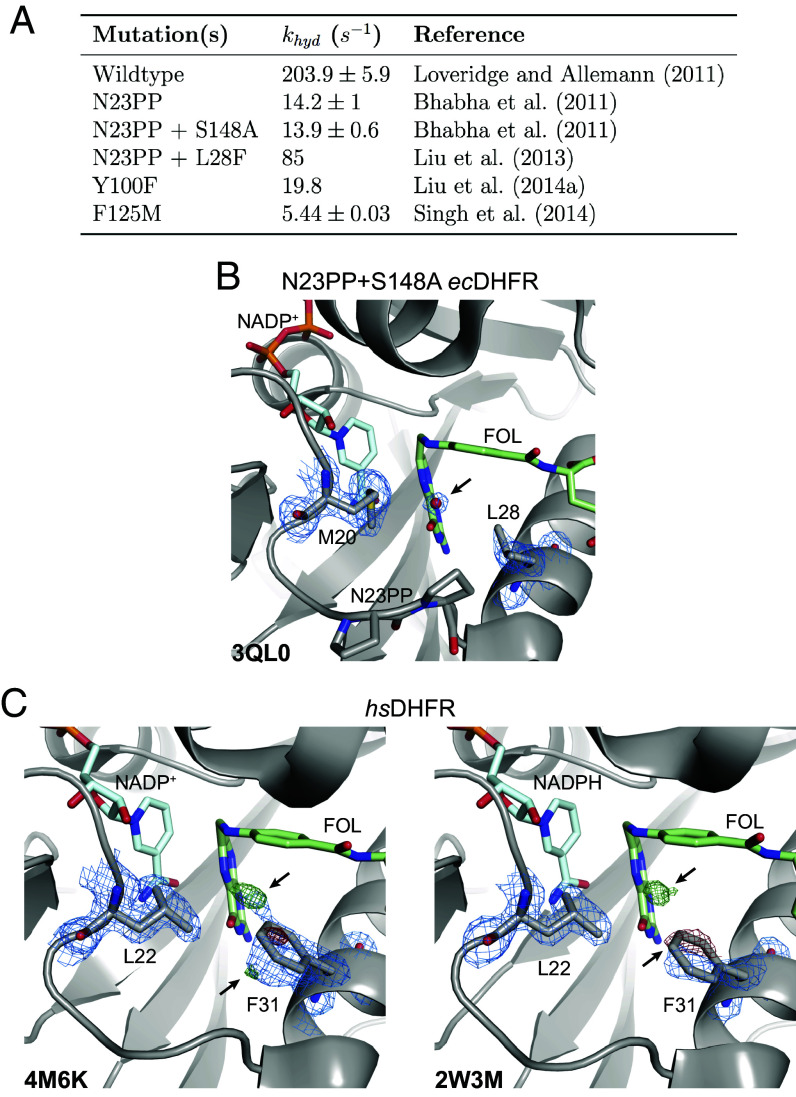
Fig. 7.
Functional importance and conservation of solvent gating in DHFR. (A) The rate of hydride transfer, , for selected mutants of ecDHFR. (B) The structure of the N23PP/S148A mutant of ecDHFR (PDB: 3QL0) shows well-supported density for an ordered water in the map (blue mesh; 1.5). (C) Structures of human DHFR (PDB: 4M6K and PDB: 2W3M, molecule B) have unmodeled density consistent with partial-occupancy water within 3.5 Å of the N5 nitrogen of FOL and evidence of an alternate rotamer for Phe31 (; green/red mesh; ). A single rotamer is supported for Leu22 in the maps (blue mesh; 1.0) suggesting that Phe31 instead serves as the solvent-gating residue in the human enzyme. Although only molecule B is presented for the 2W3M deposited structure, similar features are observed in both protein molecules of the asymmetric unit.