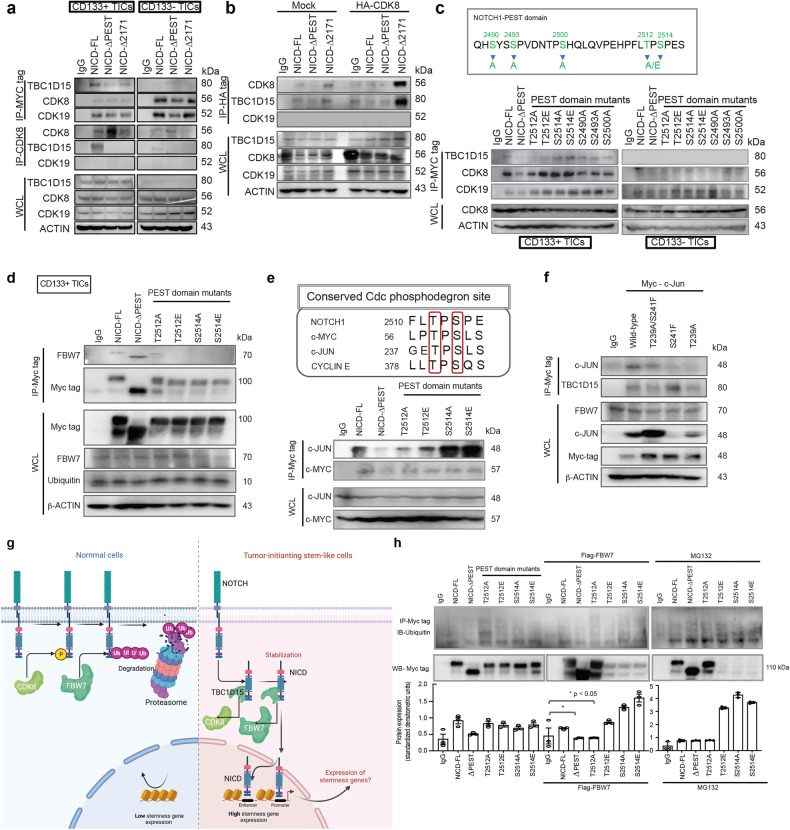
Fig. 5. TBC1D15 sequesters the NOTCH1 PEST domain to inhibit the CDK8-mediated T2512 phosphorylation of the NOTCH1 PEST domain, which is needed for recruitment of the E3 ligase FBW7 and NOTCH1 ubiquitination.
a CD133(+) and CD133(-) TICs transfected with myc-NICD-FL or NICD deletion mutants (∆PEST and ∆2171) were analyzed for the expression of the TBC1D15, CDK8 and CDK19 proteins by co-IP–western blotting. b CD133(+) TICs cotransfected with HA-CDK8, myc-NICD-FL, or NICD deletion mutants (∆PEST and ∆2171) were analyzed for the expression of the TBC1D15, CDK8 and CDK19 proteins by co-IP–western blotting. c Top-Representative phosphorylation sites for mutations in the PEST domain of NICD. Bottom-Co-IP–Western blot analysis of the interactions of TBC1D15 with PEST domain mutants in CD133(+) and CD133(-) TICs. d Co-IP–Western blot analysis of the interactions of FBW7 with T2512/S2514 mutants of the PEST domain in CD133(+) TICs. e (Top) Representative images showing the sequence alignment of the NOTCH1, CYCLIN E, c-MYC, and c-JUN FBW7 phosphodegrons. (Bottom) Co-IP–Western blot analysis of the interactions between c-JUN, c-MYC and mutants of the PEST domain in CD133(+) TICs. f Co-IP–Western blot analysis of the interactions between FBW7, c-JUN and mutants of c-JUN in CD133(+) and CD133(-) TICs. g Hypothetical model of the mechanisms by which TBC1D15 interacts with NOTCH to stabilize NOTCH1 in CD133(+) TICs and TBC1D15 inhibits NICD phosphorylation in CD133(+) TICs. h Ubiquitination assay of the anti-MYC immunoprecipitate in CD133(+) TICs treated with the proteasome inhibitor MG132 after transfection with the indicated plasmids. The bar graphs show the mean ± S.D. of three independent experiments.