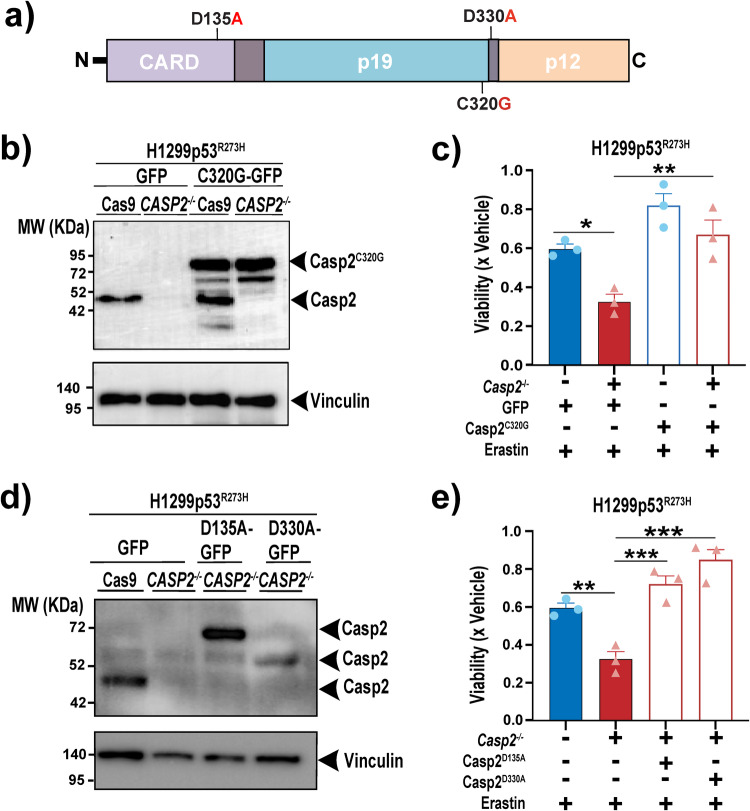
Fig. 4. The catalytic activity of caspase-2 is not required to execute its function in protecting mut-p53 cancer cells against ferroptosis.
a Diagram of caspase-2 consisting of prodomain or caspase recruitment domain (CARD), long subunit (p19), and small subunit (p12) along with the specific mutation sites introduced. b Immunoblot analysis of caspase-2 in H1299p53R273H Cas9 and H1299p53R273H-CASP2−/− cells transfected with a GFP-tagged catalytically inactive caspase-2-C320G (Casp2C320G/ C320G-GFP) expression plasmid or control plasmid (GFP). The higher MW of the ectopic Casp2C320G is because of the GFP tag. Vinculin is shown as the loading control. c Viability in H1299p53R273H Cas9 and H1299p53R273H-CASP2−/− cells ectopically expressing Casp2C320G or GFP 24 h post-treatment with erastin (2 μM) normalized to vehicle-treated cells. d Immunoblot analysis of caspase-2 in H1299p53R273H Cas9 cells transfected with GFP control plasmid, and H1299p53R273H-CASP2−/− cells transfected with GFP-tagged caspase-2-D135A (Casp2D135A/D135A-GFP) and caspase-2-D330A (Casp2D330A/D330A-GFP) expression plasmids or GFP control plasmid. Vinculin is shown as the loading control. The 60-kDa band in Casp2D330A mutant cells represents a partially processed form of caspase-2-GFP protein [5, 6]. e Viability in H1299p53R273H Cas9 cells and H1299p53R273H-CASP2−/− cells ectopically expressing Casp2D135A and Casp2D330A at 24 h post-treatment with erastin (2 μM) normalized to vehicle-treated cells. c, e Data represented as mean ±s.e.m. from three independent experiments. One-way ANOVA with Bonferroni’s post hoc test was used to estimate significant differences in c, e. P-values are indicated with *P < 0.05, **P < 0.01, and ***P < 0.001.