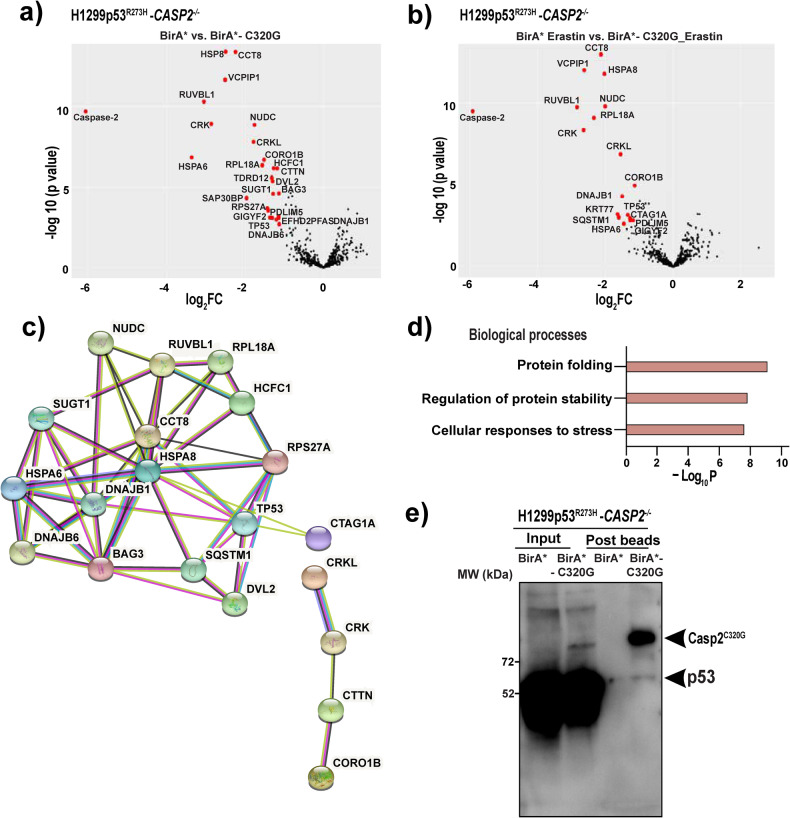
Fig. 5. Identification of novel caspase-2 interacting proteins underpinning ferroptotic cell death in mut-p53 cancer cells.
Volcano plot illustrating the log2-fold-changes of biotinylated proteins for BirA* control vs. BirA*-Casp2C320G for a untreated samples and b erastin-treated samples. Five biological replicates per group were prepared for MS analysis. Proteins were deemed to exhibit differential expression if the log2-fold-change in protein expression was ≥1-fold and an adjusted P-value ≤ 0.05. c Protein–protein interaction network among the top 28 significant caspase-2 interacting proteins, based on STRING annotations. Edges indicate a range of protein–protein associations (physical and functional) such as experimentally determined (pink), curated databases (cyan), text mining (light green), co-expression (black), and protein homology (purple). Disconnected nodes (n = 8 proteins) in the network are not shown. d Functional enrichment for biological processes (P-value < 0.01) associated with caspase-2 interacting proteins as determined by Metascape. e Co-immunoprecipitation and immunoblotting for mut-p53 in H1299p53R273H-CASP2−/− cells expressing BirA* control and BirA*-Casp2C320G before and after pull-down with streptavidin agarose beads under basal conditions.