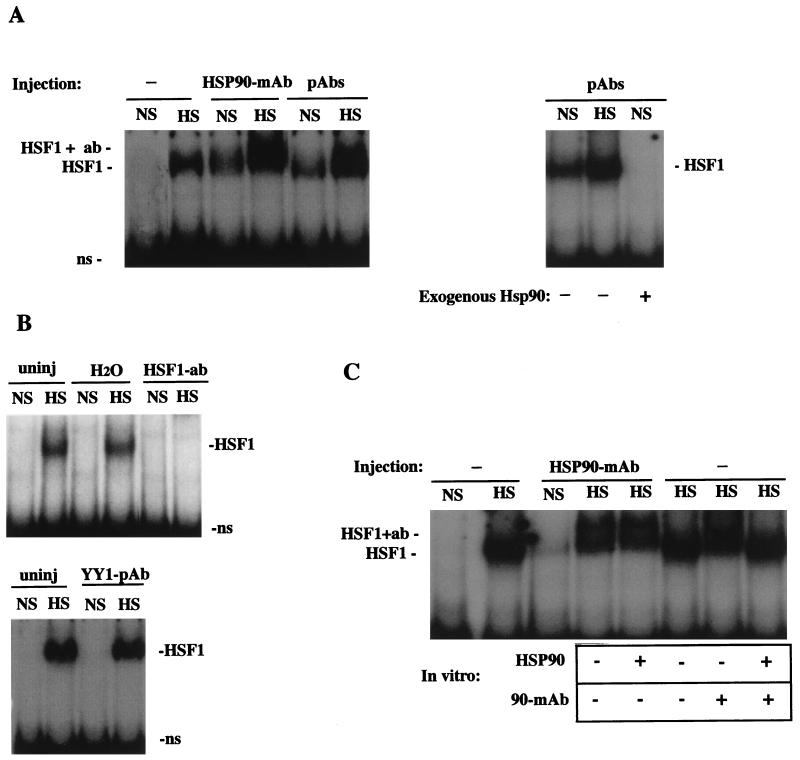
FIG. 4.
HSP90 Abs activate the HSE-binding activity of HSF1 under nonstress conditions. (A) Left, gel mobility shift assay of uninjected oocytes (−) and HSP90 Ab-injected oocytes (MAb or PAb) that were incubated at nonshock temperatures (NS) or heat shocked for 1 h (HS). The positions of heat-activated or Ab-activated HSF1-HSE complexes (HSF1) and HSP90 Ab-supershifted complexes (HSF1 + ab) are indicated. Right, gel mobility shift assay showing the effects of coinjected bovine HSP90 on the formation of HSF1-HSE complexes. In the third lane (+HSP90), 50 ng of purified bovine HSP90 was injected into oocytes 2 h after injection of HSP90 PAbs, and extracts were made following incubation at a nonshock temperature for a further 1 h. ns, nonspecific binding. (B) Bottom, gel mobility shift assay of oocytes injected with PAbs against YY1 (Santa Cruz; see Materials and Methods). Top, comparison of the activation of HSF1 in uninjected (uninj) and sham-injected (H2O) or HSF1 antiserum-injected (HSF1 PAb) (55) oocytes. (C) Recognition of HSF1 by microinjected HSP90 Abs occurred in vivo. A gel mobility shift assay was performed with uninjected (−) or HSP90 MAb-injected oocytes. In some lanes, HSP90 MAb or purified bovine HSP90 was added to extracts as indicated below the panel. HSP90 (1 μg) was added to Ab-injected oocytes at the time of homogenization (fifth lane from left), and HSP90 MAb or protein (1 μg) was added to uninjected heat-shocked oocyte extracts just prior to DNA-binding reactions (seventh and eighth lanes from left).