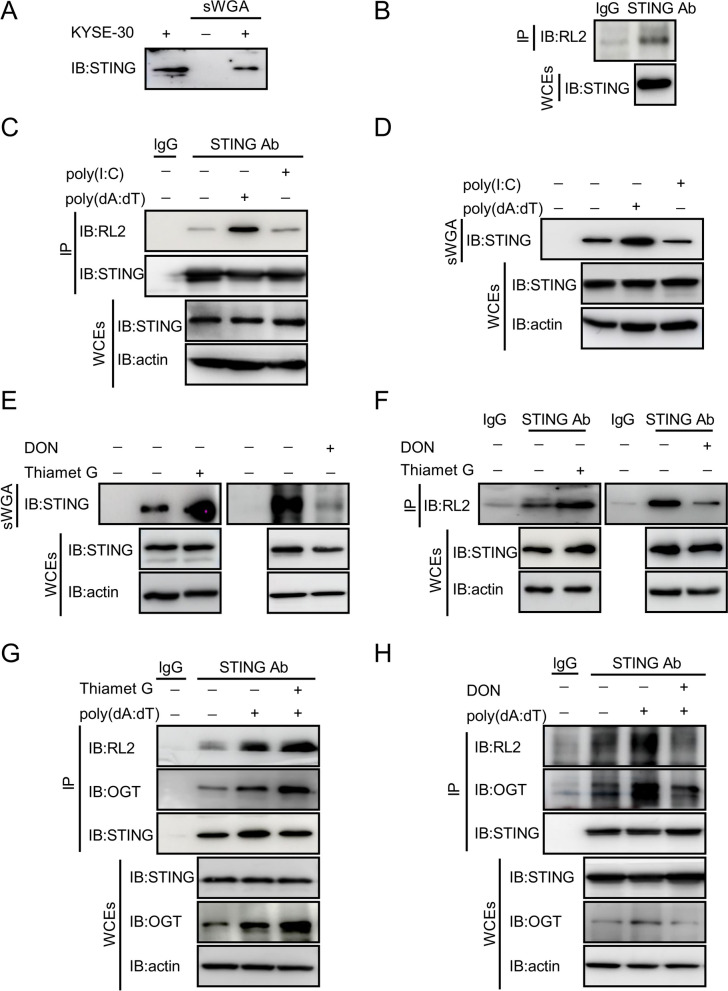
Fig. 3.
STING is O-GlcNAcylated. A O-GlcNAcylated proteins in KYSE-30 cells were pulled down with sWGA beads. STING was detected with an anti-STING antibody from abcam. B Immunoprecipitated STING was assessed for O-GlcNAcylation with a specific anti-O-GlcNAc antibody, RL2. C KYSE-30 cells were transduced with poly(dA:dT) (2 μg/mL) or poly(I:C) (4 μg/mL) for 16 h. STING in whole cell extracts (WCEs) was immunoprecipiated with an anti-STING antibody from abcam. O-GlcNAcylated STING was detected with an anti-O-GlcNAc monoclonal antibody, RL2. D WCEs of KYSE-30 cells pretreated with poly(dA:dT) (2 μg/mL) or poly(I:C) (4 μg/mL) for 16 h were isolated. O-GlcNAcylated proteins were pulled down with sWGA beads. STING in the pull-down complexes was detected with immunoblotting. WCEs were also used for the detection of STING with immunoblotting. Actin serves as a loading control. E O-GlcNAcylated proteins in KYSE-30 cells treated with TMG (10 μM) or DON (10 μM) for 12 h were pulled down with sWGA beads. STING in the pull-down complexes was detected with immunoblotting. WCEs were also analyzed with immunoblotting for the expression of STING and actin. Actin serves as a loading control. F STING in WCEs from KYSE-30 cells treated with TMG (10 μM) or DON (10 μM) for 12 h was immunoprecipiated with an anti-STING antibody. The O-GlcNAcylated STING was detected with an anti-O-GlcNAc monoclonal antibody, RL2. WCEs were also analyzed with immunoblotting for the expression of STING and actin. Actin serves as a loading control. G and H KYSE-30 cells were treated with TMG (G) or DON (H) and poly(dA:dT) (2 μg/mL) for 16 h. STING was immunoprecipitated with anti-STING antibody from abcam. RL2, OGT, and STING in the complex and in the input were detected with immunoblotting. Data are representatives from 3 independent experiments