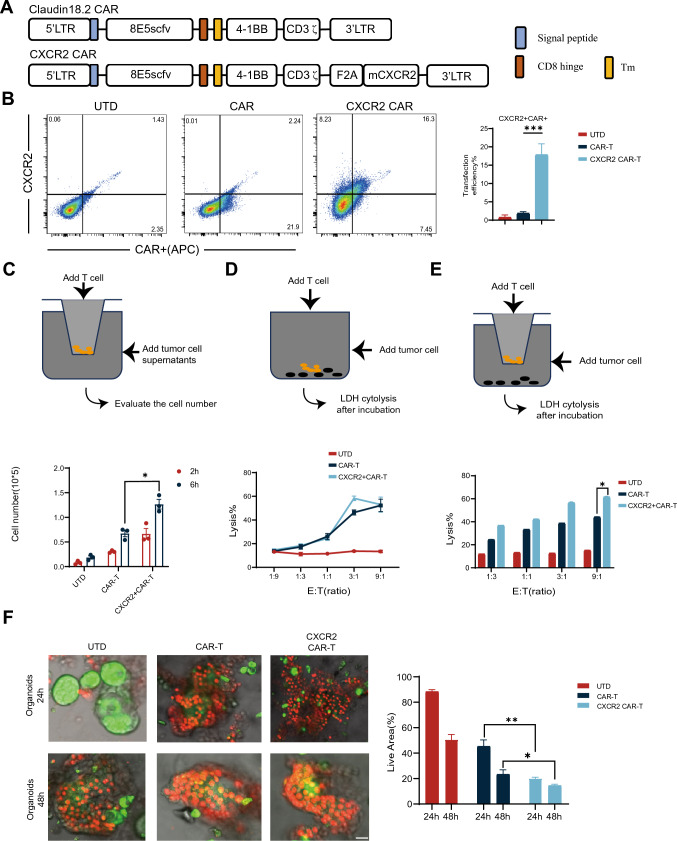
Fig. 2.
Generation of CLDN18.2-specific CAR-T cells co-expressing CXCR2. A This panel provides a schematic representation of components of the Claudin18.2-targeted conventional and CXCR2 CARs. The construct includes an extracellular antigen recognition domain, a transmembrane domain (TM), an intracellular segment of mouse 4-1BB costimulatory molecules, and a mouse CD3-ζ chain. B Transduction efficiency and CXCR2 expression in both CAR and CXCR2 CAR were determined by Fluorescence-Activated Cell Sorting (FACS) using T cells sourced from C57BL/6 mice. UTD served as negative controls. C CAR-T cells and CXCR2 CAR-T cells were introduced into the upper compartment of a Transwell plate. KPC cell culture supernatant, filtered to remove cell debris, was added to the lower compartment of the plate. The number of cells that migrated to the lower chamber was quantified either 2 or 6 h later. D CAR-T cells were co-incubated with KPC cells at varying effector-to-target (E:T) ratios for a duration of 18 h. Cell lysis was determined using standard non-radioactive cytotoxicity assays. E CAR-T cells and KPC cells were incubated together at varying E:T ratios for 18 h in both the upper and lower regions of a Transwell plate. A matrix gel was used to simulate the infiltration process across a filter mesh membrane. Cell lysis was evaluated using a standard non-radioactive cytotoxicity assay. F Representative fluorescent staining images of organoid effects by CAR-T. CAR-T cells were co-cultured with pancreatic cancer patient-derived organoid models. The cytotoxic effects of CAR-T cells were evaluated using confocal microscopy and AO/PI staining at 24 and 48 h time points. Scale bars, 50 μm. Data are expressed as mean ± SEM from triplicate experiments. Statistical significance is indicated as follows: P < 0.05, P < 0.01, **P < 0.001