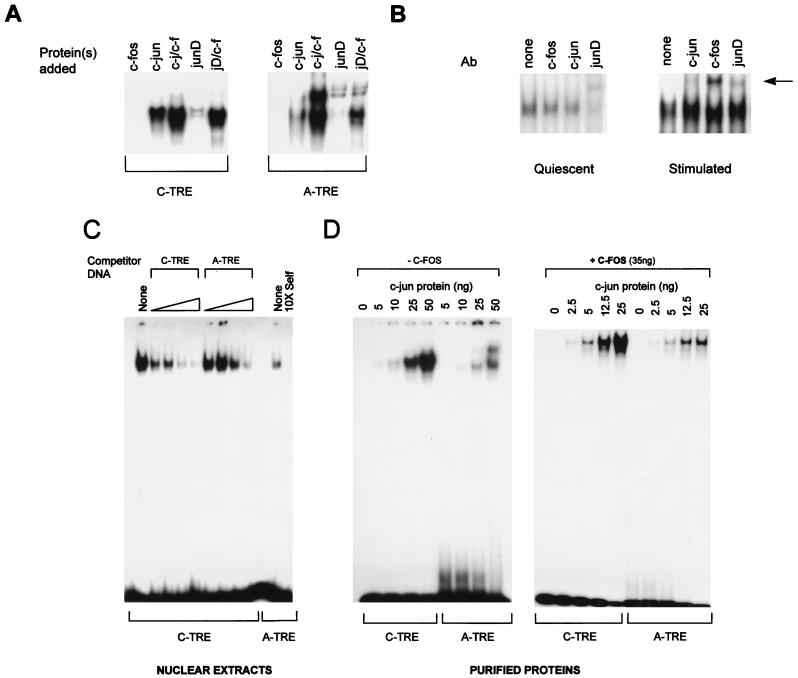
FIG. 2.
(A) In vitro binding of junD to the A-TRE is greatly enhanced in the presence of c-fos protein. Gel shift experiments were used to compare the binding of purified c-fos–c-jun (50 ng of total protein) and in vitro-translated junD (1 μl) to the C- and A-TRE probes. Only the specific AP-1–TRE complexes are shown. (B) Presence of junD in cardiocyte AP-1–A-TRE complexes and recruitment of c-fos to the complex following growth stimulation. Nuclear extracts prepared from quiescent (96 h in serum-free medium) or TPA-treated (100 ng/ml, 45 min) ventricular cardiocytes (10 or 4 μg of protein, respectively) were used in gel shift analyses, in the absence or presence of 1 μl of antibody (Ab) to either c-jun, junD, or c-fos. The supershifted complexes are arrowed. Results are similar when cardiocytes are stimulated with c-fos-inducing agents like serum (not shown). (C) The C-TRE has a higher affinity for AP-1 than the A-TRE. Increasing quantities of unlabeled oligonucleotides were used to compete the binding of HeLa cell nuclear extracts (4 μg) to a C-TRE probe in gel shift analysis. Competitors were at 10-, 25-, 50-, and 100-fold molar excess unless otherwise indicated. Binding on the A-TRE is shown for comparison. (D) The A-TRE has a lower affinity for purified AP-1 homo- or heterodimers. C- and A-TRE probes were analyzed by gel shift assay after incubation with increasing amounts of purified c-jun protein, in the absence (left) or presence (right) of a fixed amount of c-fos (35 ng). The probes were of similar specific activity. The top band seen over the A-TRE is also present in the reticulocyte lysates but not in nuclear extracts from the various cells tested.