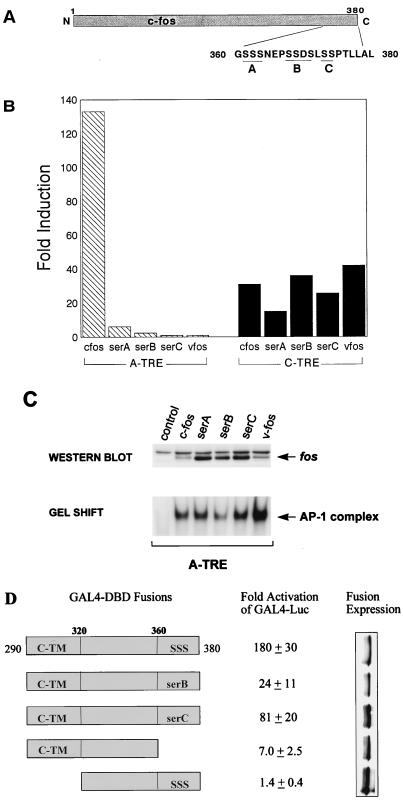
FIG. 4.
The presence of C-terminal phosphorylation sites in c-fos is critical for activation of the A-TRE. (A) Schematic of the c-fos protein showing positions of the C-terminal serine residues. (B) F9 cells were cotransfected with an A-TRE (3×) or C-TRE (1×) plasmid (3 μg) and expression vectors encoding c-jun in the presence of wild-type or mutant c-fos or v-fos (5 μg in total). The c-fos mutants replace serine with alanine residues in either the A, B, or C phosphorylation site of the c-fos C terminus (39). Data are duplicates from a representative experiment (out of four) and are represented as fold induction over activity obtained using the pRSV-neo control plasmid. (C) c-fos serine mutants are expressed in cells and cause a similar increase in AP-1 binding compared to wild-type c-fos. 293T cells were plated in 100-mm-diameter dishes and transfected with 30 μg of expression vector encoding c-fos or mutant; nuclear extracts (100 μg) prepared from these cells were Western blotted to detect protein levels or used in gel shift experiments (15 μg) to examine AP-1 binding activity. A polyclonal fos antibody was used to detect c-fos and the various mutants. (D) GAL4 fusion experiments implicate a role for the last 20 amino acids of c-fos in transactivation. HeLa cells were cotransfected with a GAL4 reporter plasmid (2 μg) and either a vector encoding GAL4 1-147 (DBD) or one of various GAL4-fos fusions (200 ng) shown. After 36 h in low serum (0.5%), cells were harvested and extracts were assayed for luciferase activity. Results (n = 4, one typical experiment) are expressed as activity relative to the control vector GAL4 1-147.