FIGURE 4.
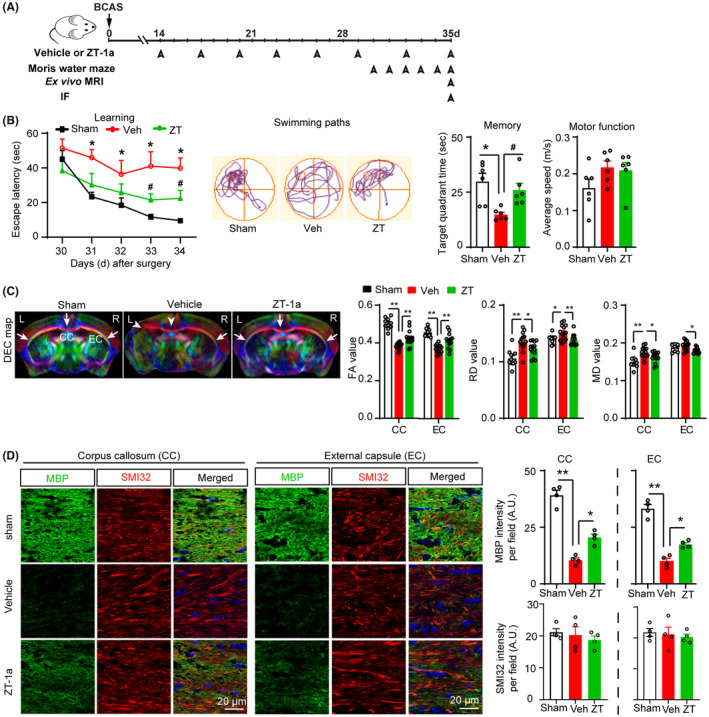
SPAK inhibitor ZT‐1a prevents memory impairments and protects white matter microstructure integrity and MBP loss after BCAS. (A) Post‐BCAS administration of vehicle (Veh) or SPAK inhibitor ZT‐1a (5 mg/kg, i.p., during 14–35 days after BCAS, every 3 days), Morris water maze test, ex vivo MRI, and immunofluorescence (IF). (B) Compared to Veh‐treated BCAS mice, ZT‐a treatment reduces the escape latency times and swimming path, and increases target quadrant time. Data are mean ± SEM; two‐way repeated‐measures ANOVA, Tukey's post hoc test; n = 6, *p < 0.05 versus sham, # p < 0.05 versus Veh. (C) Representative images of DEC maps of ex vivo brain from sham, vehicle (Veh)‐treated, and ZT‐1a (ZT)‐treated mice at 5 weeks post‐BCAS detected by MRI‐DTI. Bar graphs show quantitative analyses of fractional anisotropy (FA), radial diffusivity (RD), and mean diffusivity (MD) of CC and EC. Data are mean ± SEM; one‐way ANOVA, Tukey's post hoc test; n = 7 (data points represent values from both hemispheres), *p < 0.05, **p < 0.01. (D) Changes in myelin basic protein (MBP) and nonphosphorylated neurofilament heavy chain (SMI32) in CC and EC of brains in B–C. Quantitative analyses of MBP and SMI32 immunofluorescence. Data are mean ± SEM; one‐way ANOVA, Tukey's post hoc test; n = 3–4, *p < 0.05, **p < 0.01.
