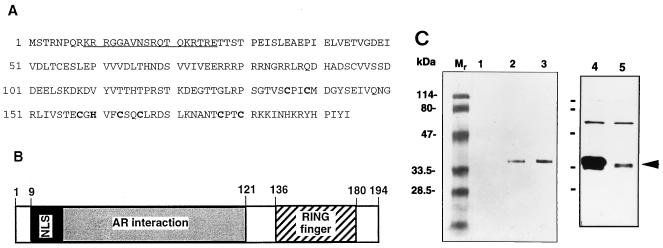
FIG. 1.
Characteristics of SNURF. (A) Predicted amino acid sequence of rat SNURF (GenBank accession no. AF022081). Cysteine and histidine residues in the consensus RING finger motif (C3HC4-type) presumably involved in zinc complexing are high-lighted, and the potential bipartite NLS is underlined. (B) Schematic structure of SNURF showing regions for bipartite NLS, AR interaction domain, and the RING finger structure. (C) Expression of SNURF protein in COS-1 cells. COS-1 cells were transfected by electroporation with pFLAG-SNURF (30 μg) (lanes 2 and 3), empty pFLAG-CMV-2 expression vector (lane 1), pcDNA-SNURF (15 μg) (lane 4), or empty pcDNA3.1 (lane 5). Cells were harvested 48 h after the transfection and then solubilized and analyzed by immunoblotting with the M2 monoclonal antibody against the FLAG epitope (lanes 1 to 3) as described in Materials and Methods or with an antiserum raised against SNURF in rabbits (1:2,500 dilution) and ECL detection reagents (Amersham) (lanes 4 and 5). The arrowhead depicts the mobility of the SNURF protein.