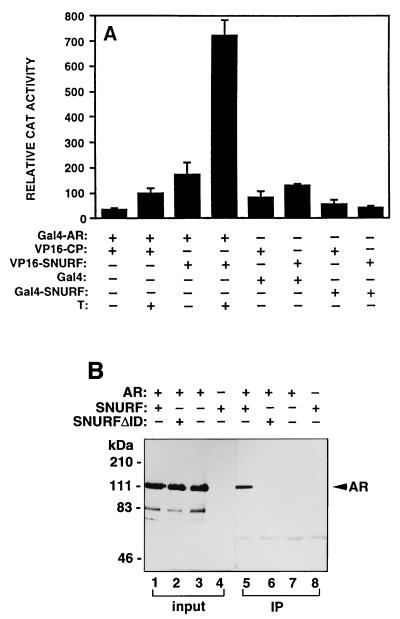
FIG. 6.
Interaction between AR and SNURF in mammalian cells. (A) The ability of rAR (residues 3 to 902) fused to the DBD of Gal4 (Gal4-AR) to interact with VP16 AD fused to SNURF residues 20 to 177 (VP16-SNURF) or to polyomavirus coat protein (VP16-CP) was examined in CV-1 cells by assaying chloramphenicol acetyltransferase (CAT) activity from the reporter plasmid pG5CAT. Cells (2.3 × 105 cells/35-mm dish) were transfected with 1.5 μg of each chimeric expression vector and 3 μg of pG5CAT reporter by using DOTAP transfection reagent. At 18 h after transfection, the medium was changed to one containing charcoal-stripped 2% (vol/vol) FBS in the presence (+) or absence (−) of 100 nM testosterone (T), and the cells were incubated for an additional 30 h. Interaction between VP16-SNURF and Gal4-SNURF was also examined. Transcriptional activity is expressed as the relative CAT activity corrected for protein concentration. The mean ± standard error values for at least three separate experiments are shown. The CAT activity derived from the interaction between hormone-bound Gal4-AR and VP16-SNURF corresponds to ca. 25% of that of a positive control; between p53 and large T antigen, tethered to Gal4 and VP16, respectively (34). (B) AR and SNURF are physically associated in COS-1 cells. COS-1 cells were transfected by electroporation with pFLAG-SNURF or pFLAG-SNURFΔID and pSG5-rAR as indicated. After a 30-h culture in the presence of 100 nM testosterone, whole-cell extracts were prepared and subjected to immunoprecipitation (IP) with mouse monoclonal anti-FLAG antibody. Immunoprecipitated proteins were analyzed by immunoblotting with a rabbit anti-AR antibody. Lanes 1 to 4 (input) represent portions of the cell extracts (5%) that were subjected to immunoblotting without prior immunoprecipitation.