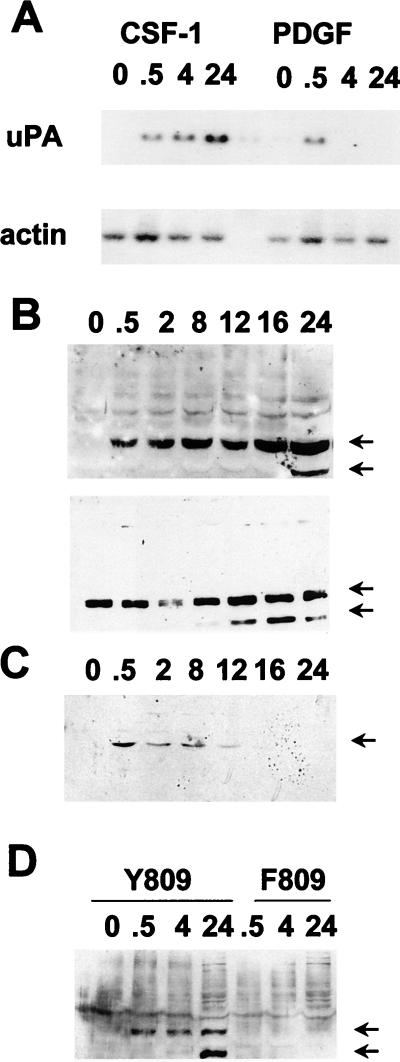
FIG. 2.
Kinetics of uPA mRNA induction and phosphorylation of ets-2 at threonine 72 in response to CSF-1/c-fms signaling in NIH 3T3 cells. (A) Northern blot of total RNA isolated from NIH 3T3 cells expressing c-fms following stimulation with CSF-1 and PDGF (2 ng/ml) for the times indicated (in hours) and probed with a mouse uPA probe (upper panel) or a γ-actin probe (lower panel). (B) Nuclear protein extracts were prepared from NIH 3T3 cells expressing c-fms following stimulation with CSF-1 for the times indicated (in hours). Extracts were run on a 10% SDS gel and Western blotted with the anti-pT72 ets-2 antibody (upper panel). The top arrow indicates the predicted location of ets-2 (54 kDa), and the lower arrow indicates a second ets-2-related band (45 kDa). The same samples in panel B were run on a second gel and probed with a nondiscriminating ets-2 antibody directed against amino acids 60 to 167 (bottom panel). (C) Nuclear protein extracts prepared from NIH 3T3 cells stimulated with 2 ng of PDGF (BB isoform) per ml for the times indicated (in hours) were analyzed by Western analysis with the anti-pT72 antibody, as described above. Only the 54-kDa form of ets-2 was detected (arrow). (D) NIH 3T3 cells expressing wild-type c-fms Y809 (lanes 1 to 4) or the c-fms F809 protein (lanes 5 to 7) were stimulated for increasing periods of time with CSF-1 as indicated (in hours). Nuclear protein extracts were prepared and analyzed by SDS-polyacrylamide gel electrophoresis (10% polyacrylamide gel) followed by Western blotting with the anti-phosphopeptide T72 ets-2 antibody.