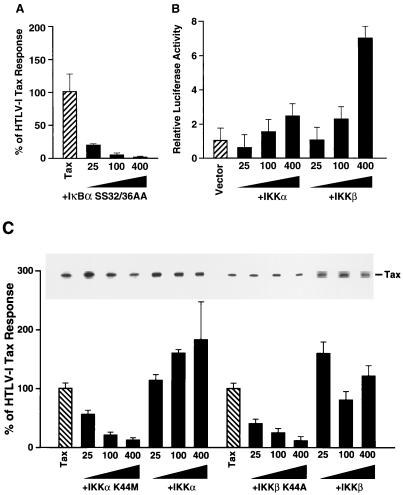
FIG. 2.
Functional analysis of the roles of IKKα and IKKβ in HTLV-1 Tax induction of NF-κB. (A) IκBα mutant HA-IκBα SS32/36AA, which fails to undergo stimulus-coupled phosphorylation and degradation, blocks Tax activation of NF-κB in human 293 cells. Cells were transfected with 1 μg of Tax expression vector, 200 ng of κB-TATA-luciferase, 20 ng of 6RZ (β-galactosidase reporter), and 25, 100, or 400 ng of HA-IκBα SS32/36AA. The total DNA concentration was held constant at 4 μg by supplementation with the parental pCMV4 vector. To control for differences in transfection efficiency, luciferase activity was normalized to β-galactosidase activity. The results presented represent the means (± standard deviations) derived from at least three independent transfections. All values were first calculated as fold induction relative to cells transfected with reporter plasmids only. These values were then expressed as percentages of the response obtained with Tax alone. (B) Transfection of 293 cells with various amounts of wild-type IKKα-T7 and IKKβ-FLAG, 200 ng of κB-luciferase reporter plasmid, and 20 ng of 6RZ. Activity is expressed as arbitrary light units normalized to β-galactosidase activity. Error bars depict standard deviations obtained from triplicate determinations. (C) Functional effects of wild-type and kinase-deficient forms of IKKα-T7 and IKKβ-FLAG on HTLV-1 Tax activation of κB-luciferase activity. Human 293 cells were transfected with 1 μg of Tax expression vector, 200 ng of κB-TATA-luciferase plasmid, 20 ng of 6RZ, and 25, 100, or 400 ng of either the wild-type IKKs or the kinase-deficient mutant of IKKα-T7 (K44M) or IKKβ-FLAG (K44A). κB-dependent luciferase activity is depicted as a percentage of the response obtained in the presence of Tax alone as described for panel A. The inset depicts the level of Tax protein expressed in each of the cultures assessed by immunoblotting with an anti-Tax antibody.