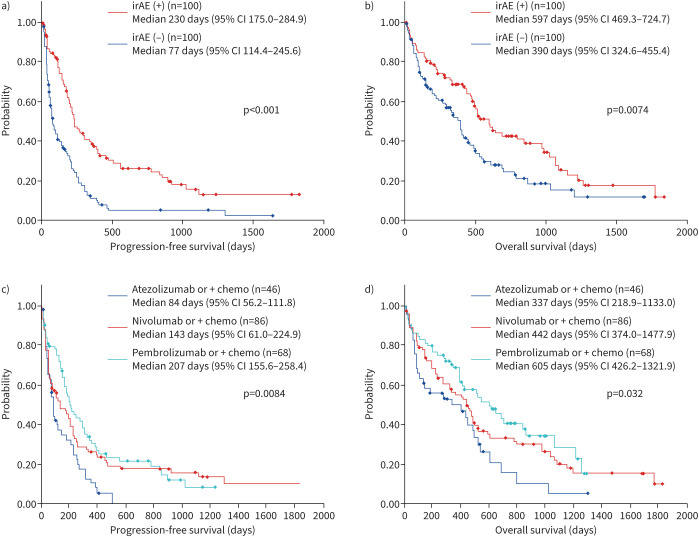
FIGURE 3.
Efficacy of immune checkpoint inhibitor therapy in primary lung cancer patients with underlying interstitial pneumonia (n=200). a, b) Prognosis was significantly better in patients with irAEs (n=100) compared with patients without irAEs (n=100) (post-ICI treatment, median PFS 230 days versus 77 days, median OS 597 days versus 390 days). c, d) A significant difference was observed in prognosis of patients treated with different ICI agents (PFS, p=0.0084; OS, p=0.032): pembrolizumab monotherapy or combination therapy (median PFS 207 days; median OS 605 days), nivolumab monotherapy or combination therapy (median PFS 143 days; median OS 442 days) and atezolizumab monotherapy or combination therapy (median PFS 87 days; median OS 337 days). ICI: immune checkpoint inhibitor; irAE: immune-related adverse event; OS: overall survival; PFS: progression-free survival.