Abstract
INTRODUCTION
Older adults experiencing subjective cognitive decline (SCD) have a higher risk of dementia. Reducing this risk through behavioral interventions, which can increase emotional well‐being (mindfulness and compassion) and physical activity, is crucial in SCD.
METHODS
SCD‐Well is a multicenter, observer‐blind, randomized, controlled, superiority trial. Three hundred forty‐seven participants (mean [standard deviation] age: 72.7 [6.9] years; 64.6% women) were recruited from memory clinics in four European sites to assess the impact of an 8‐week caring mindfulness‐based approach for seniors (CMBAS) and a health self‐management program (HSMP) on mindfulness, self‐compassion, and physical activity.
RESULTS
CMBAS showed a significant within‐group increase in self‐compassion from baseline to post‐intervention and both a within‐ and between‐group increase to follow‐up visit (24 weeks). HSMP showed a significant within‐ and between‐group increase in physical activity from baseline to post‐intervention and to follow‐up visit.
DISCUSSION
Non‐pharmacological interventions can differentially promote modifiable factors linked to healthy aging in older adults with SCD.
Keywords: aging, Alzheimer's disease, dementia, mindfulness, non‐pharmacological interventions, physical activity, self‐compassion
1. BACKGROUND
The increase in life expectancy is associated with a higher prevalence of age‐related health conditions, 1 and higher dementia cases, expected to reach 153 million by 2050. 2 Subjective cognitive decline (SCD) is defined as the subjective perception of decline in cognition, even though scores on cognitive tests remain in the normal range. 3 Patients with SCD are at higher risk of developing dementia, 4 , 5 and more likely to experience mental health difficulties, including anxiety 6 and depressive symptoms, 7 which are, in turn, associated with an increased risk of cognitive impairment. 8 , 9
It has been suggested that about 40% of dementia cases could be prevented by acting on modifiable risk factors. 10 Past studies have related low education, smoking, diabetes, social isolation, and physical inactivity, 10 , 11 , 12 but also psychological factors, such as depression 13 , 14 and anxiety, 15 to poorer cognitive outcomes, lower brain integrity, and/or greater dementia risk. This suggests that a reduction of such lifestyle and psycho‐affective modifiable risk factors, and an increase of protective factors, constitute a powerful target to promote health and well‐being in aging, but also to delay dementia onset and/or reduce Alzheimer's disease cases. 16
Recently, mindfulness and compassion meditation have been proposed to promote mental health, well‐being, and cognition in the context of healthy aging, and to reduce psycho‐affective risk factors for dementia. 16
Mindfulness refers to paying attention to emotions, thoughts, or inner experiences in the present moment without judgment. 17 Training in mindfulness has been shown to help develop attention, emotion regulation, and psychological well‐being, as well as to reduce stress, anxiety, and prevent recurrence of mood disorders. 16 , 18 , 19 Trait mindfulness is associated with greater acceptance and openness. 20 Researchers have hypothesized that sustained and regular practice of mindfulness meditation can positively affect aging by improving mental health and perceived well‐being, 21 as well as cognition, 22 , 23 and neuroplastic changes in brain regions sensitive to aging. 24
Self‐compassion, defined as a feeling of kindness toward oneself, having a sense of common humanity, and having an awareness of negative thoughts and feelings without over‐identification, 25 may represent a valuable psychological resource for positive aging by improving subjective and psychological well‐being. 26 , 27 It has been further demonstrated that self‐compassion can have a beneficial impact on age‐related thoughts and that it is correlated with better mental health. 28 Moreover, self‐compassion has been related to a lower incidence rate of mental health disorders and symptoms (e.g., worry, depression, anxiety), 27 , 29 and with a range of positive psychological outcomes, including health‐promoting behaviors, 30 motivation, life satisfaction, optimism, and happiness 31 in older adults. Despite the growing interest in mindfulness 24 and self‐compassion, 32 research on the impact of meditation training on these outcomes in older adults is largely lacking. On this basis, self‐compassion may be of great importance for older adults and may help improve psychological interventions to promote healthy aging.
Exercise, as demonstrated by different reviews, is one of the most robust lifestyle changes associated with increased health and a decreased risk of cognitive impairment. 33 , 34 , 35 , 36 Physical activity has many benefits for physical and mental functions and reversing some effects of chronic disease. 37 It has a positive impact on general health and quality of life, 38 mental health, 39 , 40 as well as on healthy aging. 41 Additional evidence suggests that a physically active life is associated with better brain health 34 , 42 and better cognition, 43 independent functioning, 44 and psychological health for older adults experiencing cognitive decline. 45 Therefore, interventions that increase physical activity in older adults are seen as a promising way to promote healthy aging.
1.1. Objectives
The SCD‐Well trial is part of the “Medit‐Ageing” project (public name: Silver Santé Study) funded through the European Union as part of the Horizon 2020 program. The present study is a secondary analysis of the SCD‐Well trial. 46 The trial's primary outcome was the mean change in trait anxiety symptoms after an 8‐week caring mindfulness‐based approach for seniors (CMBAS) intervention, compared to a health self‐management program (HSMP). In the primary outcome, participants reported a reduction in trait anxiety after both interventions, maintained at 6‐month follow‐up, with no differences observed between the two groups. 47 Moreover, in a secondary analysis, we observed beneficial effects of both trainings on cognition, demonstrating a modest improvement in global cognition, which was maintained at 6‐month follow‐up with no difference between the two interventions. 48
The present study aims to extend these findings by assessing the relative impact of CMBAS and HSMP interventions on psychological and lifestyle behaviors associated with healthy ageing, and whether any changes are maintained at 6‐month follow‐up. Based on the assumption that training in mindfulness is the crucial active component of the CMBAS intervention, HSMP was selected as the comparison condition, which is structurally equivalent to the mindfulness‐based training. HSMP is designed to improve lifestyle behaviors without targeting compassion and self‐compassion. We hypothesized that the two interventions would have differential effects on our outcomes, with a greater improvement in mindfulness and self‐compassion after CMBAS, and a greater improvement in physical activity after HSMP.
RESEARCH IN CONTEXT
Systematic review: A systematic review of the literature about non‐pharmacological interventions to target protective factors related to delayed onsets of dementia (e.g., physical activity, mindfulness, and compassion), showed that multicenter clinical randomized controlled trials (RCT) are missing in older adults with subjective cognitive decline (SCD).
Interpretation: The current multicenter RCT SCD‐Well shows that 8 weeks of regular mindfulness and compassion training increase self‐compassion and that 8 weeks of regular health self‐management training increase physical activity, with both changes being maintained at 6‐month follow‐up.
Future direction: Future studies are needed to test the clinical significance of these findings and to compare such intervention effects to passive control groups.
2. MATERIALS AND METHODS
2.1. Trial design, setting, and participants
SCD‐Well is a European multicenter, observer‐blind, controlled trial comparing the effects of an 8‐week CMBAS to an 8‐week HSMP. The trial was conducted in four European memory clinics (London, UK; Cologne, Germany; Lyon, France; Barcelona, Spain) and included physician‐referred and self‐referring patients. After pre‐screening, participants underwent a diagnostic assessment at the screening visit (V0) to determine eligibility, criteria reported in Table 1 (see Marchant et al. 46 ). Eligible participants proceeded to the baseline visit (V1) and were randomized into HSMP or CMBAS groups. Post‐intervention assessment occurred at the end of the 8‐week intervention (V2), with a follow‐up at 24 weeks post‐randomization (V3; 6 months after V1). Each visit included biological and behavioral assessments, encompassing mindfulness, self‐compassion, and physical activity questionnaires.
TABLE 1.
Eligibility criteria.
| Inclusion criteria | Exclusion criteria |
|---|---|
|
|
2.2. Interventions
The CMBAS followed the format of the mindfulness‐based stress reduction program, including a pre‐class interview, eight weekly 2 hour group sessions, and a half‐day meditation practice in the sixth week. Each session involved group meditation (sitting and walking), sharing, and teaching. To incorporate mindfulness skills into daily life, participants were encouraged to engage daily in both formal and informal guided meditations. Based on previous work by Zellner Keller et al., 53 CMBAS was specifically designed to address the needs of older adults, aiming to develop mindfulness, kindness, and compassion to cope with challenges related to aging.
HSMP was selected as the comparison condition. It followed the same format and structure as the CMBAS and was matched in administration, dosage, and duration. Specifically, it consisted of a pre‐class meeting with the facilitator, eight weekly group‐based sessions of 2 hour duration, a half‐day of practice after the sixth session of the program, and home practices. The program was based on a published manual for guidance on living with chronic conditions 54 that has been previously adapted and validated in a population with SCD. 55 Every session of the program covered different topics (e.g., self‐management, problem solving, sleep, stress, exercise, eating, and planning for the future). Participants were provided with information about these topics and engaged in group exercises and discussions about them. They were asked to create and implement “action plans” to promote engagement in activities to improve health and well‐being on 6 days per each week.
Each site had two clinically trained facilitators, one for each intervention group. They were provided with a specific intervention protocol, instructions, and a day‐long training about their respective intervention before starting the study. Facilitators completed checklists to monitor the fidelity of treatment delivery. 56
2.3. Outcomes
All outcomes were collected at pre‐ (V1), post‐intervention (V2; 8 weeks after V1), and at follow‐up (V3; 6 months after V1) visits. In the present study, we assessed the relative impact of CMBAS and HSMP on mindfulness, self‐compassion, and physical activity.
2.3.1. Mindfulness
Mindfulness was measured with the 39‐item self‐report Five Facet Mindfulness Questionnaire (FFMQ). 57 Items are rated on a 5‐point Likert scale, ranging from 1 (never or very rarely true) to 5 (very often or always true). 58 A higher score indicates a higher mindfulness level.
2.3.2. Self‐compassion
Self‐compassion was measured with the Self‐Compassion Scale–Short Form (SCS‐SF). This self‐report questionnaire has 12 items rated on a 5‐point Likert scale (0 = “Almost never” to 5 = “Almost always”) to record how often one behaves kindly and caringly toward oneself in difficult life situations. 59 A higher score corresponds to higher levels of self‐compassion.
2.3.3. Physical activity
Physical activity was evaluated with the Physical Activity Scale for the Elderly (PASE), a brief self‐report survey, designed to assess physical activity in older adults over the last week. 60 It uses frequency, duration, and intensity levels of activity over the previous week to assign a total score, ranging from 0 to 793, with higher scores indicating greater engagement in physical activities. 61
2.4. Statistical considerations
2.4.1. Sample size
The sample size measurement was conducted based on the primary outcome. 47 Specifically, as the trait State‐Trait Anxiety Inventory has no absolute cut‐off levels, the sample size was based on the effect size (i.e., the ratio between the expected interarm differences from the common standard deviation). With a minimum effect size of 0.50, 62 64 participants per group (128 total) were needed to demonstrate a significant difference in the primary endpoint in a t test with 80% power and a two‐sided type I error of 5%. A greater number of participants were recruited in anticipation that a small proportion of volunteers would drop out of the trial and to provide sufficient power for secondary analyses.
2.4.2. Statistical methods
Descriptive statistics were calculated for the sample's demographics and baseline measures. Linear mixed models (LMMs) were used to assess the effect of intervention assignment on outcomes over time. All models included age at baseline (years), education level (years), sex, trial site, trial group, and Mini‐Mental State Examination (MMSE), as well as random participant intercepts. Time was modeled by the inclusion of a factor variable for visit (coded as V1, V2, or V3). Intervention effects were compared through the inclusion of an interaction term between visit and trial group. Within‐group changes were also examined. The LMM used all available data for analysis, including participants for whom outcome values were missing for one or two visits (e.g., due to dropout). The LMM achieves this by interpolating missing values through the subject‐pooled covariance matrix, based on a missing at random (MAR) assumption, which assumes that missing values can be recovered from observed values.
Analyses were conducted in R v.4.2.1 (www.R‐project.org). LMMs were fit using the package lme4 v.1.1‐30; P values for LMMs were obtained via lmerTest v.3.1‐3. Post hoc analyses to obtain LMM‐adjusted means and 95% confidence intervals (CIs) for each group/outcome/time point, as well as change (Δ) in scores within and across groups, were run using the emmeans package v.1.8.2. The evaluation of the visit‐by‐group interaction effect included both an omnibus test (analysis of variance [ANOVA]; to test for between‐group differences in the trajectory of the outcomes across all visits), as well as post hoc contrasts evaluating the between‐group effects from V1 to V2, and V1 to V3. We opted to conduct post hoc contrasts even when the ANOVA was not significant, as we hypothesized that different mechanisms might affect the outcomes from V1 to V2, versus V1 to V3. That is, for V1 to V2, the recency of interventions might be most relevant, whereas from V1 to V3, change in the outcome may reflect the cumulative effects of engagement with the interventions over time. For all analyses, uncorrected P values are reported and were deemed statistically significant at < 0.05.
2.5. Safety and study monitoring
SCD‐Well is registered on ClinicalTrials.gov (NCT03005652) and adheres to Consolidated Standards of Reporting Trials (CONSORT) of non‐pharmacologic treatment guidelines. 63 The sponsor established a trial steering committee according to Good Clinical Practice guidelines with the responsibility to provide oversight on the conduct of the trial, advise on scientific credibility on behalf of the sponsor and the funder, and assess the progress of the trial.
For more details on data management, monitoring, dissemination and access, and study governance (blinding, safety, auditing) see Marchant et al. (their supporting material 1). 46 Briefly, the local study coordinator dedicated to this observer‐blinded study oversaw data management at the first level under the responsibility of the principal investigator.
3. RESULTS
3.1. Participant flow and baseline characteristics
Recruitment took place from March 23, 2017, to January 25, 2018. Data collection was completed on September 18, 2018. Figure 1 shows the flow of participants through the study. Among the 147 participants who took part in the study and completed the questionnaires, 95 (65%) were female and 52 (35%) were male, with a mean age of 72.2 years. The participants were randomized after the inclusion visit (V1) with a 1:1 allocation (stratified by site), resulting in a sample of 73 participants in CMBAS and 74 in HSMP. The CMBAS and HSMP intervention groups did not differ on demographic characteristics or in their engagement in the interventions (Table 2). Table 3 shows the average observed values for each outcome by group and visit.
FIGURE 1.
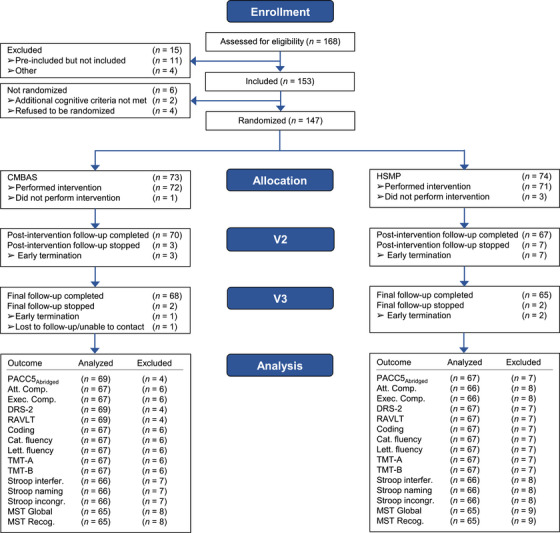
Consort flow diagram of enrolment and randomization to CMBAS and HSMP interventions. CMBAS, caring mindfulness‐based approach for seniors; HSMP, health self‐management program.
TABLE 2.
Participant characteristics.
| Characteristics | Total sample (n = 147) | CMBAS (n = 73) | HSMP (n = 74) | |
|---|---|---|---|---|
| Age, years | 72.7 ± 6.9 | 72.1 ± 7.6 | 73.3 ± 6.2 | |
| Sex | Female/male ratio | 95/52 (65/35) | 47/26 (64/36) | 48/26 (65/35) |
| Education, years | 13.6 ± 3.6 | 13.9 ± 3.8 | 13.4 ± 3.4 | |
| Ethnicity | White | 142 (97) | 69 (94) | 73 (99) |
| Other | 5 (3) | 4 (6) | 1 (1) | |
| Recruitment center | London, UK | 28 (19) | 14 (19) | 14 (19) |
| Lyon, France | 40 (27) | 20 (27) | 20 (27) | |
| Cologne, Germany | 39 (26) | 19 (26) | 20 (27) | |
| Barcelona, Spain | 40 (27) | 20 (27) | 20 (27) | |
| Employment status | Retired | 123 (85) | 58 (82) | 65 (88) |
| Not retired | 19 (15) | 12 (18) | 7 (12) | |
| MMSE | 28.8 ± 1.1 | 28.7 ± 1.2 | 28.9 ± 1.0 | |
| McNair scale | 52.50 ± 20.77 | 53.92 ± 21.34 | 51.07 ± 20.07 |
Note: Data are presented as mean ± standard deviation or numbers (%).
Abbreviations: CMBAS, caring mindfulness‐based approach for seniors; HSMP, health self‐management program; MMSE, Mini‐Mental State Examination.
TABLE 3.
Scores for mindfulness, self‐compassion, and physical activity by intervention condition.
| Baseline visit (V1) | Post‐intervention visit (V2) | Follow‐up visit (V3) | |||||
|---|---|---|---|---|---|---|---|
| CMBAS Mean (SD) | HSMP Mean (SD) | CMBAS Mean (SD) | HSMP Mean (SD) | CMBAS Mean (SD) | HSMP Mean (SD) | Potential range | |
| Mindfulness | 51.41 (7.26) | 51.91 (7.38) | 51.14 (8.57) | 51.81 (7.85) | 51.05 (8.53) | 52.15 (8.19) | 0–195 |
| Self‐ compassion | 37.65 (6.92) | 38.74 (7.48) | 39.53 (7.19) | 39.90 (7.15) | 40.51 (6.60) | 39.13 (6.82) | 0–60 |
| Physical activity | 129.74 (74.86) | 117.99 (64.23) | 127.21 (71.70) | 137.02 (64.29) | 127.61 (63.88) | 140.62 (65.44) | 0–793 |
Abbreviations: CMBAS, caring mindfulness‐based approach for seniors; HSMP, health self‐management program; SD, standard deviation.
3.2. Mindfulness
The ANOVA for the visit by group interaction was not significant, indicating that the change in mindfulness scores across visits did not differ between interventions (F[2, 238] = 0.17, P = 0.84). Furthermore, within‐group comparisons (Table 4) showed that mindfulness scores did not change in the CMBAS and HSMP group neither from V1 to V2 nor from V1 to V3.
TABLE 4.
Estimated within‐group and between‐group differences in changes in mindfulness, self‐compassion, and physical activity.
| CMBAS (n = 73) | HSMP (n = 74) | |||
|---|---|---|---|---|
| Within‐group estimated change | Between‐group difference in change | |||
| Estimate (95% CI) | Estimate (95% CI) | Estimate (95% CI) | P value | |
| Mindfulness | ||||
| V1 to V2 | −0.19 (−1.81; 1.43) | −0.17 (−1.78; 1.45) | 0.02 (−2.27; 2.31) | 0.98 |
| V1 to V3 | −0.34 (−1.97; 1.29) | 0.27 (−1.31; 1.84) | 0.60 (−1.67; 2.87) | 0.60 |
| Self‐compassion | ||||
| V1 to V2 | 2.00 (0.38; 3.61) * | 0.69 (−0.94; 2.33) | −1.31 (−3.60; 0.99) | 0.26 |
| V1 to V3 | 2.57 (0.96; 4.19) ** | 0.20 (−1.40; 1.81) | −2.37 (−4.65; −0.10) * | 0.04 |
| Physical activity | ||||
| V1 to V2 | −7.43 (−21.03; 6.17) | 18.74 (4.44; 33.04) * | 26.17 (6.43; 45.91) * | <0.01 |
| V1 to V3 | −2.79 (−16.98; 11.39) | 25.74(11.82; 39.66) *** | 28.54 (8.66; 48.41) * | <0.01 |
Note: For between‐group differences, positive differences favor HSMP, whereas negative differences favor CMBAS. All analyses included covariates for sex, age, education years, baseline MMSE, site, visit, group, the visit by group interaction, as well as random participant intercepts. Significant effects appear in bold.
Abbreviations: CI, confidence interval; CMBAS, caring mindfulness‐based approach for seniors; HSMP, health self‐management program; MMSE, Mini‐Mental State Examination; V1, baseline visit; V2, post‐intervention visit; V3, follow‐up visit.
P < 0.05.
P < 0.005.
P < 0.001.
3.3. Self‐compassion
The ANOVA for the visit by group interaction was not significant, indicating that the change in self‐compassion scores across visits did not differ between interventions (F[2, 240] = 2.12, P = 0.12). However, post hoc tests comparing the change in scores from V1 to V3 between groups favored the CMBAS over HSMP (estimated change [95% CI]: −2.37 [−4.65; −0.10], P = 0.04), and not from V1 to V2 (−1.31 [95% CI: −3.60; 0.99], P = 0.26; Table 4 and Figure 2). Within‐group analyses showed an increase in self‐compassion scores from V1 to V2 (estimated change [95% CI]: 2.00 [0.38; 3.61], P = 0.02), and also from V1 to V3 (2.57 [95% CI: 0.96; 4.19], P < 0.01) in the CMBAS group. In contrast, within‐group scores did not change in the HSMP group from V1 to V2 (0.69 [95% CI: −0.94; 2.33], P = 0.40) or from V1 to V3 (0.20 [95% CI: −1.40; 1.81], P = 0.80).
FIGURE 2.
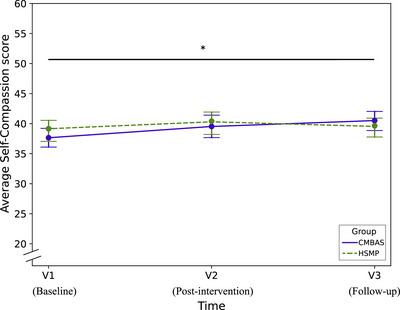
This data‐based plot shows the evolution of average self‐compassion scores from baseline (V1) to post‐intervention (V2) and follow‐up (V3) for each intervention condition. The y axis represents the mean score on the SCS‐SF questionnaire from 20 to 60 (observed range in SCD‐Well: 37 to 41). V1, baseline visit; V2, post‐intervention visit after 8 weeks; V3, follow‐up visit 24 weeks post‐intervention. Asterisk corresponds to between‐group significance. * P < 0.05. CMBAS, caring mindfulness‐based approach for seniors; HSMP, health self‐management program; SCS‐SF, Self‐Compassion Scale–Short Form.
3.4. Physical activity
The ANOVA for the visit by group interaction was significant, indicating that the change in physical activity scores across visits differed between interventions (F(2, 240) = 5.06, P < 0.01). Post hoc tests comparing the change in scores from V1 to V2 between groups favored the HSMP over CMBAS (estimated difference in change: 26.17 [95% CI: 6.43; 45.91], P < 0.01), and also from V1 to V3 (28.54 [95% CI: 8.66; 48.41, P < 0.01; Table 4 and Figure 3). Within‐group analyses showed that physical activity increased from V1 to V2 (estimated change: 18.74 [95% CI: 4.44; 33.04], P = 0.01) and from V1 to V3 (25.74 [95% CI: 11.82; 39.66], P < 0.01) in the HSMP group, while no significant changes were observed in the CMBAS group from V1 to V2 (−7.43 [95% CI: −21.03; 6.17], P = 0.28) or V1 to V3 (−2.79 [95% CI: −16.98; 11.39], P = 0.70).
FIGURE 3.
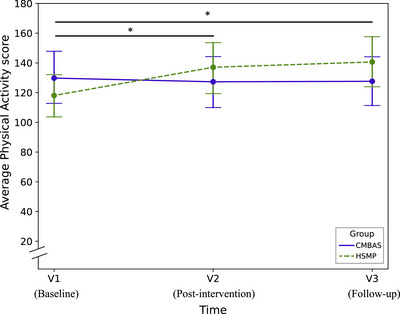
This data‐based plot shows the evolution of average physical activity scores from baseline (V1) to post‐intervention (V2) and follow‐up (V3) for each intervention condition. The y axis represents the mean score on the PASE questionnaire from 20 to 200 to allow for a better visualization (maximum range: from 0 to 793, observed range in SCD‐Well: 117 to 141. V1, baseline visit; V2, post‐intervention visit after 8 weeks; V3, follow‐up visit 24 weeks post‐intervention. Asterisks correspond to between‐group significance. * P < 0.05. CMBAS, caring mindfulness‐based approach for seniors; HSMP, health self‐management program; PASE, Physical Activity Scale for the Elderly.
4. DISCUSSION
To the best of our knowledge, this is the first longitudinal randomized controlled trial testing the impact of a mindfulness and compassion‐based intervention and a HSMP, both specifically adapted for older adults with SCD, on psychological and lifestyle factors, namely mindfulness, self‐compassion, and physical activity. While no changes in mindfulness were observed, intervention‐specific benefits were found in self‐compassion and physical activity. More specifically, the CMBAS intervention improved self‐compassion post‐intervention and at 6‐month follow‐up. On the other hand, the HSMP increased engagement in physical activities post‐intervention and this increase was maintained at 6‐month follow‐up.
4.1. Mindfulness
Contrary to our hypothesis we did not observe a change in mindfulness after the CMBAS program. This result contrasts with previous research showing greater increase in mindfulness after a mindfulness‐based intervention both in the general population 22 , 64 , 65 , 66 and in older adults. 22 , 67 Several factors could explain this result. First, the CMBAS intervention explicitly focused on cultivating compassion and self‐compassion and less time was spent on mindfulness practice. Second, the 8 weeks of intervention may not have provided sufficient time for participants to develop mindfulness skills, 68 particularly given their age and the potential decline in cognitive abilities. A longer intervention period would allow them more time to embed the skills they learned and practice independently. Moreover, self‐report–based measures of mindfulness are prone to introspection limitations, which can be challenging. 69 Future studies are needed to test the dosage of mindfulness training. Furthermore, future studies could use behavioral tasks 19 to evaluate whether mindfulness skills are objectively increased over the course of a mindfulness‐based intervention.
4.2. Self‐compassion
Self‐compassion increased from pre‐ to post‐intervention and to 6‐month follow‐up in the CMBAS group, but not in the HSMP group. Moreover, while between‐group differences in change in self‐compassion need to be interpreted with caution (given the non‐significant ANOVA), scores followed a significantly more salutary trajectory in the CMBAS versus HSMP group from V1 to V3 (but not from V1 to V2). Our results are in accordance with previous studies demonstrating an increased level in self‐compassion directly after an 8‐week mindfulness‐based intervention. However, these studies were conducted in a younger adult sample. 70 , 71 , 72 Moreover, the increase in self‐compassion was seen also at the 6‐month follow‐up visit, suggesting that the benefits of the mindfulness intervention were embedded by participants in their life.
Given self‐compassion's association with life satisfaction and self‐care, 31 , 73 sense of connectedness with others, 74 and psychological resilience, 75 the increase in self‐compassion in older adults with SCD observed after the CMBAS training indicates that this intervention may be one promising avenue to promote healthy aging.
4.3. Physical activity
A small number of studies have shown promising results regarding the potential of health education interventions to promote physical activity engagement. 76 , 77 , 78 However, longitudinal and randomized controlled studies in older adults have been lacking. Our study demonstrated a beneficial impact of a HSMP on physical activity engagement in people with SCD immediately after the intervention and at 6‐month follow‐up. This is of great importance given the association of physical activity with mental and physical health, including measures such as self‐esteem, quality of life, life expectancy, and mortality. 79 Also, the increment in physical activity is of particular importance for older adults with SCD, 12 because it has also been linked to a lower risk of dementia. 10 Our study indicates that the HSMP holds promise as an intervention to promote healthy aging via improving physical activity. However, it was not designed to address other lifestyle changes and future studies are needed to test for other improvements.
The findings from this study extend those from the primary outcome paper, which observed an improvement in subclinical anxiety symptoms maintained at follow‐up after both the CMBAS and HSMP. 47 The present study highlights that in addition to joint effects, the two interventions also have a differential effect on modifiable risk factors for dementia.
For both interventions, the effects were maintained at 6‐month follow‐up. This was also the case for changes in anxiety 47 ; therefore, it may be that the increment in self‐compassion 80 , 81 and in physical activity 82 , 83 , 84 was a possible mechanism through which anxiety improved in CMBAS and HSMP groups, respectively. While the follow‐up period is relatively short, such maintained benefits suggest that even after only 8 weeks of intervention, lasting effects can be observed in older adults with SCD.
5. STRENGTHS AND LIMITATIONS
This study has strengths and limitations. The strength is that we conducted a randomized controlled clinical trial with a longitudinal 6‐month follow‐up, a large sample of older adults with SCD meeting strict eligibility criteria, and a well‐matched active comparator intervention. This allowed us to assess the causal, differential, and longer term impact of CMBAS and HSMP interventions on mindfulness, self‐compassion, and physical activity in older adults with SCD.
This study has several limitations. First, all outcomes reported here are based on self‐reports, which can be affected by recall bias and social desirability. Future studies might consider using more objective measures of self‐compassion and physical activity. Second, the intervention and follow‐up were relatively short. While there were statistically significant improvements in self‐compassion and physical activity, changes were small, and the clinical significance of these changes is unknown. Future studies with longer interventions and longer follow‐up tests are needed. Finally, it will be important for future research to incorporate passive control groups to clearly establish the causal role of mindfulness practices in promoting holistic health in older adults.
6. CONCLUSION
This study provides evidence regarding the immediate and sustained effects of a CMBAS intervention on self‐compassion and of a HSMP on physical activity. This work adds to the growing body of evidence that non‐pharmacological interventions can impact modifiable risk factors relevant for well‐being and dementia risk in an older adult population with SCD. Importantly, our study may suggest that health‐care practitioners in community, primary care, or clinical settings could make use of targeted interventions for participants at risk for dementia.
CONFLICT OF INTEREST STATEMENT
T.B. has received honoraria for workshops on MBI and is the co‐author of a book on mindfulness‐based cognitive therapy published by Guilford Press. O.K. received honoraria for research, training, and consulting related to meditation. All the other authors, Y.D., T.W., M.S., A.L., G.C., N.L.M., and J.G., have no conflicts to declare. Author disclosures are available in the supporting information.
COLLABORATORS
The SCD‐WELL Medit‐Ageing Research Group includes Florence Allais, Eider Arenaza Urquijo, Romain Bachelet, Viviane Belleoud, Beatriz Bosch, Maria Pilar Casanova, Pierre Champetier, Léa Chauveau, Anne Chocat, Fabienne Collette, Nina Coll‐Padros, Sophie Dautricourt, Robin De Flores, Vincent De La Sayette, Floriane Delphin‐Combe, Harriet Demnitz‐King, Hélène Espérou, Séverine Fauvel, Francesca Felisatti, Eric Frison, Karine Goldet, Sacha Haudry, Frank Jessen, Pierre Krolak‐Salmon, Elizabeth Kuhn, Brigitte Landeau, Maria Leon, Dix Meiberth, Florence Mezenge, José Luis Molinuevo, Hendrik Mueller, Cassandre Palix, Géraldine Poisnel, Géraldine Rauchs, Leslie Reyrolle, Eric Salmon, Yamna Satgunasingam, Ann‐Katrin Schild, Hilde Steinhauser, Edelweiss Touron, Anne‐Laure Turpin, Zuzana Walker, and Miranka Wirth.
CONSENT STATEMENT
Written informed consent was secured from all participants after the procedures had been fully explained to them and prior to trial participation. The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
Supporting information
Supporting Information
ACKNOWLEDGMENTS
Many people helped in this study. The authors would like to thank Ben Meuleman for his help in statistics, all the Medit‐Ageing Research Group, and the participants in the study. SCD‐Well was sponsored by the Institut National de la Santé et de la Recherche Médicale (Inserm). T.B., O.K., A.L., N.L.M., and G.C., received support for the Medit‐Ageing project funded through the European Union in Horizon 2020 program related to the call PHC22 “Promoting Mental Well‐Being in the Aging Population” and under grant agreement No. 667696. N.L.M. was supported by a Senior Fellowship from the Alzheimer's Society (AS‐SF‐15b‐002). J.G. was supported by a Young Researcher Grant 2019‐2022 from the Fondation Alzheimer and Fondation de France. A.L. and G.C. were supported by Fondation d'Entreprise MMA, des Entrepreneurs du Futur and MMA and by Institut National de la Santé et de la Recherche Médicale (Inserm). O.K. received funding from the Secrétariat d'État à la formation, à la recherche et à l'innovation (SEFRI) under contract no. 15.0336 in the context of the European project “Medit‐Ageing.” G.C. received funding from Fondation Alzheimer, Programme Hospitalier de Recherche Clinique, Fondation Alzheimer, Agence Nationale de la Recherche, Région Normandie, Association France Alzheimer et maladies apparentées, Fondation Vaincre Alzheimer, Fondation Recherche Alzheimer. The funders had no role in the study design, data acquisition, data analysis, data interpretation, or writing.
D'elia Y, Whitfield T, Schlosser M, et al. Impact of mindfulness‐based and health self‐management interventions on mindfulness, self‐compassion, and physical activity in older adults with subjective cognitive decline: A secondary analysis of the SCD‐Well randomized controlled trial. Alzheimer's Dement. 2024;16:e12558. 10.1002/dad2.12558
Natalie L. Marchant, Julie Gonneaud, and Olga Klimecki are considered last authors.
Contributor Information
Olga Klimecki, Email: olga.klimecki@uni-jena.de.
The Medit‐Ageing group:
Florence Allais, Eider Arenaza Urquijo, Romain Bachelet, Viviane Belleoud, Beatriz Bosch, Maria Pilar Casanova, Pierre Champetier, Léa Chauveau, Anne Chocat, Fabienne Collette, Nina Coll‐Padros, Sophie Dautricourt, Robin De Flores, Vincent De La Sayette, Floriane Delphin‐Combe, Harriet Demnitz‐King, Hélène Espérou, Séverine Fauvel, Francesca Felisatti, Eric Frison, Karine Goldet, Sacha Haudry, Frank Jessen, Pierre Krolak‐Salmon, Elizabeth Kuhn, Brigitte Landeau, Maria Leon, Dix Meiberth, Florence Mezenge, José Luis Molinuevo, Hendrik Mueller, Cassandre Palix, Géraldine Poisnel, Géraldine Rauchs, Leslie Reyrolle, Eric Salmon, Yamna Satgunasingam, Ann‐Katrin Schild, Hilde Steinhauser, Edelweiss Touron, Anne‐Laure Turpin, Zuzana Walker, and Miranka Wirth
REFERENCES
- 1. World Health Organization . a Vital Investment. World Health. 2005:202. http://scholar.google.com/scholar?hl=en&btnG=Search&q=intitle:Preventing+Chronic+Diseases:+A+Vital+Investment#3
- 2. Nichols E, Steinmetz JD, Vollset SE, et al. Estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: an analysis for the Global Burden of Disease Study 2019. Lancet Public Health. 2022;7(2):e105‐e125. doi: 10.1016/S2468-2667(21)00249-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jessen F, Amariglio RE, Buckley RF, et al. The characterisation of subjective cognitive decline. Lancet Neurol. 2021;19(3):271‐278. doi: 10.1016/S1474-4422(19)30368-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rabin LA, Smart CM, Crane PK, et al. Subjective cognitive decline in older adults: an overview of self‐report measures used across 19 International Research Studies. J Alzheimer's Dis. 2015;48(S1):S63‐S86. doi: 10.3233/JAD-150154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mitchell AJ, Beaumont H, Ferguson D, Yadegarfar M, Stubbs B. Risk of dementia and mild cognitive impairment in older people with subjective memory complaints: meta‐analysis. Acta Psychiatr Scand. 2014;130(6):439‐451. doi: 10.1111/acps.12336 [DOI] [PubMed] [Google Scholar]
- 6. Perrotin A, La Joie R, de La Sayette V, et al. Subjective cognitive decline in cognitively normal elders from the community or from a memory clinic: differential affective and imaging correlates. Alzheimers Dement. 2017;13(5):550‐560. doi: 10.1016/j.jalz.2016.08.011 [DOI] [PubMed] [Google Scholar]
- 7. Zlatar ZZ, Muniz M, Galasko D, Salmon DP. Subjective cognitive decline correlates with depression symptoms and not with concurrent objective cognition in a clinic‐based sample of older adults. J Gerontol B Psychol Sci Soc Sci. 2017;73(7):1198‐1202. doi: 10.1093/geronb/gbw207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gulpers B, Ramakers I, Hamel R, Köhler S, Oude Voshaar R, Verhey F. Anxiety as a predictor for cognitive decline and dementia: a systematic review and meta‐analysis. Am J Geriatr Psychiatry. 2016;24(10):823‐842. doi: 10.1016/j.jagp.2016.05.015 [DOI] [PubMed] [Google Scholar]
- 9. Diniz BS, Albert S, Reynolds CF. Late‐life depression and risk of vascular dementia and Alzheimer's disease: systematic review and meta‐analysis of community‐based cohort studies. Br J Psychiatry. 2013;202(5):329‐335. doi: 10.1192/bjp.bp.112.118307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Livingston G, Huntley J, Sommerlad A, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet North Am Ed. 2020;396(10248):413‐446. doi: 10.1016/S0140-6736(20)30367-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kimura N, Aso Y, Yabuuchi K, et al. Modifiable lifestyle factors and cognitive function in older people: a cross‐sectional observational study. Front Neurol. 2019;10(APR):1‐12. doi: 10.3389/fneur.2019.00401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lautenschlager NT, Cox KL, Ellis KA, Lautenschlager NT, Cox KL, Ellis KA. Physical activity for cognitive health : what advice can we give to older adults with subjective cognitive decline and mild cognitive impairment ? Dialogues Clin Neurosci. 2019;21(1):61‐68. doi: 10.31887/DCNS.2019.21.1/nlautenschlager [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bennett S, Thomas AJ. Depression and dementia: cause, consequence or coincidence? Maturitas. 2014;79(2):184‐190. doi: 10.1016/j.maturitas.2014.05.009 [DOI] [PubMed] [Google Scholar]
- 14. Chételat G, Lutz A, Arenaza‐Urquijo E, Collette F, Klimecki O, Marchant N. Why could meditation practice help promote mental health and well‐being in aging? Alzheimers Res Ther. 2018;10(1):10‐13. doi: 10.1186/s13195-018-0388-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Santabárbara J, Lopez‐Anton R, de la Cámara C, et al. Clinically significant anxiety as a risk factor for dementia in the elderly community. Acta Psychiatr Scand. 2019;139(1):6‐14. doi: 10.1111/acps.12966 [DOI] [PubMed] [Google Scholar]
- 16. Lutz A, Chételat G, Collette F, Klimecki OM, Marchant NL, Gonneaud J. The protective effect of mindfulness and compassion meditation practices on ageing: hypotheses, models and experimental implementation. Ageing Res Rev. 2021;72:101495. doi: 10.1016/j.arr.2021.101495 [DOI] [PubMed] [Google Scholar]
- 17. Giluk TL. Mindfulness, Big Five personality, and affect: a meta‐analysis. Pers Individ Dif. 2009;47(8):805‐811. doi: 10.1016/j.paid.2009.06.026 [DOI] [Google Scholar]
- 18. Hazlett‐Stevens H, Singer J, Chong A. Mindfulness‐based stress reduction and mindfulness‐based cognitive therapy with older adults: a qualitative review of randomized controlled outcome research. Clin Gerontol. 2019;42(4):347‐358. doi: 10.1080/07317115.2018.1518282 [DOI] [PubMed] [Google Scholar]
- 19. Geiger PJ, Boggero IA, Brake CA, et al. Mindfulness‐based interventions for older adults: a review of the effects on physical and emotional well‐being. Mindfulness (N Y). 2016;7(2):296‐307. doi: 10.1007/s12671-015-0444-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bishop SR, Lau M, Shapiro S, et al. Mindfulness: a proposed operational definition. Clinical Psychology: Science and Practice. 2004;11(3):230‐241. doi: 10.1093/clipsy/bph077 [DOI] [Google Scholar]
- 21. Mahlo L, Windsor TD. Older and more mindful? Age differences in mindfulness components and well‐being. Aging Ment Health. 2021;25(7):1320‐1331. doi: 10.1080/13607863.2020.1734915 [DOI] [PubMed] [Google Scholar]
- 22. Moynihan JA, Chapman BP, Klorman R, et al. Mindfulness‐based stress reduction for older adults: effects on executive function, frontal alpha asymmetry and immune function. Neuropsychobiology. 2013;68(1):34‐43. doi: 10.1159/000350949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Whitfield T, Barnhofer T, Acabchuk R, et al. The effect of mindfulness based programs on cognitive function in adults: a systematic review and meta analysis. Neuropsychol Rev. 2022;32(3):677‐702. doi: 10.1007/s11065-021-09519-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Klimecki O, Marchant NL, Lutz A, Poisnel G, Chételat G, Collette F. The impact of meditation on healthy ageing — the current state of knowledge and a roadmap to future directions. Curr Opin Psychol. 2019;28:223‐228. doi: 10.1016/j.copsyc.2019.01.006 [DOI] [PubMed] [Google Scholar]
- 25. NEFF K. Self‐compassion: an alternative conceptualization of a healthy attitude toward oneself. Self and Identity. 2003;2(2):85‐101. doi: 10.1080/15298860309032 [DOI] [Google Scholar]
- 26. Phillips WJ, Ferguson SJ. Self‐compassion : a resource for positive aging. J Gerontol B Psychol Sci Soc Sci. 2013;68(4):529‐539. doi: 10.1093/geronb/gbs091 [DOI] [PubMed] [Google Scholar]
- 27. Homan KJ. Self‐compassion and psychological well‐being in older adults. J Adult Dev. 2016;23(2):111‐119. doi: 10.1007/s10804-016-9227-8 [DOI] [Google Scholar]
- 28. Tavares LR, Vagos P, Xavier A. The role of self‐compassion in the psychological (mal)adjustment of older adults: a scoping review. Int Psychogeriatr. 2023;35(4):179‐192. doi: 10.1017/S1041610220001222 [DOI] [PubMed] [Google Scholar]
- 29. Neff KD. Self‐compassion, self‐esteem, and well‐being. Soc Personal Psychol Compass. 2011;5(1):1‐12. [Google Scholar]
- 30. Allen AB, Goldwasser ER, Leary MR. Self‐compassion and well‐being among older adults. Self and Identity. 2012;11(4):428‐453. doi: 10.1080/15298868.2011.595082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kim C, Ko H. The impact of self‐compassion on mental health, sleep, quality of life and life satisfaction among older adults. Geriatr Nurs (Minneap). 2018;39(6):623‐628. doi: 10.1016/j.gerinurse.2018.06.005 [DOI] [PubMed] [Google Scholar]
- 32. Yeshua M, Zohar AH, Berkovich L. “Silence! The body is speaking”–a correlational study of personality, perfectionism, and self‐compassion as risk and protective factors for psychosomatic symptoms distress. Psychol Health Med. 2019;24(2):229‐240. doi: 10.1080/13548506.2018.1546016 [DOI] [PubMed] [Google Scholar]
- 33. Bherer L, Erickson KI, Liu‐Ambrose T. Physical Exercise and Brain Functions in Older Adults. J Aging Res. 2013;2013:197326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rolland Y, Abellan van Kan G, Vellas B. Physical activity and Alzheimer's disease: from prevention to therapeutic perspectives. J Am Med Dir Assoc. 2008;9(6):390‐405. doi: 10.1016/j.jamda.2008.02.007 [DOI] [PubMed] [Google Scholar]
- 35. Warburton DER, Nicol CW, Bredin SSD. Review Health benefits of physical activity : the evidence. CMAJ. 2006;174(6):801‐809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lee Y, Back JH, Kim J, et al. Erratum: systematic review of health behavioral risks and cognitive health in older adults. Int Psychogeriatr. 2010;22(2):188. doi: 10.1017/S1041610209991645 [DOI] [PubMed] [Google Scholar]
- 37. Mcphee JS, French DP, Jackson D, Nazroo J, Pendleton N, Degens H. Physical activity in older age : perspectives for healthy ageing and frailty. Biogerontology. 2016;17(3):567‐580. doi: 10.1007/s10522-016-9641-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Dougherty RJ, Boots EA, Lindheimer JB, et al. Fitness, independent of physical activity is associated with cerebral blood flow in adults at risk for Alzheimer's disease. Brain Imaging Behav. 2020;14(4):1154‐1163. doi: 10.1007/s11682-019-00068-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Maynou L, Hernández‐pizarro HM, Rodríguez ME. The association of physical (In)activity with mental health. differences between elder and younger populations: a systematic literature review. Int J Environ Res Public Health. 2021;18(9):4771. doi: 10.3390/ijerph18094771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Paluska SA, Schwenk TL. Physical activity and mental health current concepts. Sports Med. 2000;29(3):167‐180. [DOI] [PubMed] [Google Scholar]
- 41. Daskalopoulou C, Stubbs B, Kralj C, Koukounari A, Prince M, Prina AM. Physical activity and healthy ageing: a systematic review and meta‐analysis of longitudinal cohort studies. Ageing Res Rev. 2017;38:6‐17. doi: 10.1016/j.arr.2017.06.003 [DOI] [PubMed] [Google Scholar]
- 42. Liu‐Ambrose T, Barha CK, Best JR. Physical activity for brain health in older adults. Appl Physiol Nutr Metab. 2018;43(11):1105‐1112. doi: 10.1139/apnm-2018-0260 [DOI] [PubMed] [Google Scholar]
- 43. Newson R, Kemps E. Cardiorespiratory fitness as a predictor of successful cognitive ageing. J Clin Exp Neuropsychol. 2006;28(6):949‐967. doi: 10.1080/13803390591004356 [DOI] [PubMed] [Google Scholar]
- 44. Mee OG, Conn VS. Meta‐analysis of the effects of exercise interventions on functional status in older adults. Res Nurs Health. 2008;31(6):594‐603. doi: 10.1002/nur.20290 [DOI] [PubMed] [Google Scholar]
- 45. Nuzum H, Stickel A, Corona M, Zeller M, Melrose RJ, Wilkins SS. Potential benefits of physical activity in MCI and dementia. Behav Neurol. 2020;2020:7807856. doi: 10.1155/2020/7807856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Marchant NL, Barnhofer T, Klimecki OM, et al. The SCD‐Well randomized controlled trial: effects of a mindfulness‐based intervention versus health education on mental health in patients with subjective cognitive decline (SCD). Alzheimers Dement. 2018;4:737‐745. doi: 10.1016/j.trci.2018.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Marchant NL, Barnhofer T, Coueron R, et al. Effects of a mindfulness‐based intervention versus health self‐management on subclinical anxiety in older adults with subjective cognitive decline: the scd‐well randomized superiority trial. Psychother Psychosom. 2021;90(5):341‐350. doi: 10.1159/000515669 [DOI] [PubMed] [Google Scholar]
- 48. Whitfield T, Demnitz‐King H, Schlosser M, et al. Effects of a mindfulness‐based versus a health self‐management intervention on objective cognitive performance in older adults with subjective cognitive decline (SCD): a secondary analysis of the SCD‐Well randomized controlled trial. Alzheimers Res Ther. 2022;14(1):125. doi: 10.1186/s13195-022-01057-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Jessen F, Amariglio RE, Van Boxtel M, et al. A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer's disease. Alzheimers Dementia. 2014;10(6):844‐852. doi: 10.1016/j.jalz.2014.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Bondi MW, Edmonds EC, Jak AJ, et al. Neuropsychological criteria for mild cognitive impairment improves diagnostic precision, biomarker associations, and progression rates. J Alzheimers Disease. 2014;42:275‐289. doi: 10.3233/JAD-140276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Jak AJ, Bondi MW, Delano‐Wood L, et al. Quantification of five neuropsychological approaches to defining mild cognitive impairment. Am J Geriatr Psychiatry. 2009;17(5):368‐375. doi: 10.1097/JGP.0b013e31819431d5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Molinuevo JL, Rabin LA, Amariglio R, et al. Implementation of subjective cognitive decline criteria in research studies. Alzheimers Dementia. 2017;13(3):296‐311. doi: 10.1016/j.jalz.2016.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Zellner Keller B, Singh NN, Winton ASW. Mindfulness‐based cognitive approach for seniors (MBCAS): program development and implementation. Mindfulness. 2014;5(4):453‐459. doi: 10.1007/s12671-013-0262-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Lorig K, Holman H, Sobel D. Living a Healthy Life with Chronic Conditions: For Ongoing Physical and Mental Health Conditions. Bull Publishing Company; 2013. [Google Scholar]
- 55. Wetherell JL, Hershey T, Hickman S, et al. Mindfulness‐based stress reduction for older adults with stress disorders and neurocognitive difficulties: a randomized controlled trial. J Clin Psychiatry. 2017;78(7):11977. [DOI] [PubMed] [Google Scholar]
- 56. Borrelli B. The assessment, monitoring, and enhancement of treatment fidelity in public health clinical trials. J Public Health Dent. 2011;71(1):1‐21. doi: 10.1111/j.1752-7325.2011.00233.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Gu J, Strauss C, Crane C, et al. Supplemental material for examining the factor structure of the 39‐item and 15‐item versions of the five facet mindfulness questionnaire before and after mindfulness‐based cognitive therapy for people with recurrent depression. Psychol Assess. 2016;28(7):791‐802. doi: 10.1037/pas0000263.supp [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Baer RA, Smith GT, Hopkins J, Krietemeyer J, Toney L. Using self‐report assessment methods to explore facets of mindfulness. Assessment. 2006;13(1):27‐45. doi: 10.1177/1073191105283504 [DOI] [PubMed] [Google Scholar]
- 59. Raes F, Pommier E, Neff KD, Van Gucht D. Construction and factorial validation of a short form of the self‐compassion scale. Clin Psychol Psychother. 2011;18(3):250‐255. doi: 10.1002/cpp.702 [DOI] [PubMed] [Google Scholar]
- 60. Washburn RA, Smith KW, Jette AM, Janney CA. The physical activity scale for the elderly (PASE): development and evaluation. J Clin Epidemiol. 1993;46(2):153‐162. doi: 10.1016/0895-4356(93)90053-4 [DOI] [PubMed] [Google Scholar]
- 61. Logan SL, Gottlieb BH, Maitl SB, Meegan D, Spriet LL. The physical activity scale for the elderly (PASE) questionnaire; Does it predict physical health? Int J Environ Res Public Health. 2013;10(9):3967‐3986. doi: 10.3390/ijerph10093967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Chen KW, Berger CC, Manheimer E, et al. Meditative therapies for reducing anxiety: a systematic review and meta‐analysis of randomized controlled trials. Depress Anxiety. 2012;29(7):545‐562. doi: 10.1002/da.21964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Boutron I, Moher D, Altman DG, Schulz KF, Ravaud P. Extending the CONSORT statement to randomized trials of nonpharmacologic treatment: explanation and elaboration. Ann Intern Med. 2008;148(4):295‐309. www.annals.org [DOI] [PubMed] [Google Scholar]
- 64. Takahashi T, Sugiyama F, Kikai T, et al. Changes in depression and anxiety through mindfulness group therapy in Japan: the role of mindfulness and self‐compassion as possible mediators. Biopsychosoc Med. 2019;13(1):4. doi: 10.1186/s13030-019-0145-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Nyklíček I, Kuijpers KF. Effects of mindfulness‐based stress reduction intervention on psychological well‐being and quality of life: is increased mindfulness indeed the mechanism? Ann Behav Med. 2008;35(3):331‐340. doi: 10.1007/s12160-008-9030-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Song Y, Lindquist R. Effects of mindfulness‐based stress reduction on depression, anxiety, stress and mindfulness in Korean nursing students. Nurse Educ Today. 2015;35(1):86‐90. doi: 10.1016/j.nedt.2014.06.010 [DOI] [PubMed] [Google Scholar]
- 67. Lenze EJ, Hickman S, Hershey T, et al. Mindfulness‐based stress reduction for older adults with worry symptoms and co‐occurring cognitive dysfunction. Int J Geriatr Psychiatry. 2014;29(10):991‐1000. doi: 10.1002/gps.4086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Segal ZV, Williams JMG, Teasdale JD. Mindfulness‐Based Cognitive Therapy for Depression: A New Approach to Preventing Relapse. Guilford Press; 2002. [Google Scholar]
- 69. Van Dam NT, van Vugt MK, Vago DR, et al. Mind the hype: a critical evaluation and prescriptive agenda for research on mindfulness and meditation. Perspect Psychol Sci. 2018;13(1):36‐61. doi: 10.1177/1745691617709589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Birnie K, Speca M, Carlson LE. Exploring self‐compassion and empathy in the context of mindfulness‐based stress reduction (MBSR). Stress and Health. 2010;26(5):359‐371. doi: 10.1002/smi.1305 [DOI] [Google Scholar]
- 71. Joss D, Khan A, Lazar SW, Teicher MH. Effects of a mindfulness‐based intervention on self‐compassion and psychological health among young adults with a history of childhood maltreatment. Front Psychol. 2019;10(OCT):1‐13. doi: 10.3389/fpsyg.2019.02373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Wasson RS, Barratt C, O'Brien WH. Effects of Mindfulness‐Based Interventions on Self‐compassion in Health Care Professionals: a Meta‐analysis. Mindfulness. 2020;11(8):1914‐1934. doi: 10.1007/s12671-020-01342-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Karmiyati D, Wahyuningsih YP. Self‐care ability and self‐compassion: the implication toward life satisfaction of the elderly. Adv Soc Sci Humanit Res. 2019;304:372‐378. doi: 10.2991/acpch-18.2019.90 [DOI] [Google Scholar]
- 74. Leary MR, Tate EB, Adams CE, Allen AB, Hancock J. Self‐compassion and reactions to unpleasant self‐relevant events: the implications of treating oneself kindly. J Pers Soc Psychol. 2007;92(5):887‐904. doi: 10.1037/0022-3514.92.5.887 [DOI] [PubMed] [Google Scholar]
- 75. Neff KD, Kirkpatrick KL, Rude SS. Self‐compassion and adaptive psychological functioning. J Res Pers. 2007;41(1):139‐154. doi: 10.1016/j.jrp.2006.03.004 [DOI] [Google Scholar]
- 76. Greene GW, White AA, Hoerr SL, et al. Impact of an online healthful eating and physical activity program for college students. Am J Health Promot. 2012;27(2):47‐59. doi: 10.4278/ajhp.110606-QUAN-239 [DOI] [PubMed] [Google Scholar]
- 77. Harrison M, Burns CF, McGuinness M, Heslin J, Murphy NM. Influence of a health education intervention on physical activity and screen time in primary school children: “Switch Off‐Get Active.”. J Sci Med Sport. 2006;9(5):388‐394. doi: 10.1016/j.jsams.2006.06.012 [DOI] [PubMed] [Google Scholar]
- 78. Yang XH, Yu HJ, Liu MW, et al. The impact of a health education intervention on health behaviors and mental health among Chinese college students. J Am Coll Health. 2020;68(6):587‐592. doi: 10.1080/07448481.2019.1583659 [DOI] [PubMed] [Google Scholar]
- 79. Cvecka J, Tirpakova V, Sedliak M, Kern H, Mayr W, Hamar D. Physical activity in elderly. Eur J Transl Myol. 2015;25(4):249. doi: 10.4081/ejtm.2015.5280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Brown L, Huffman JC, Bryant C. Self‐compassionate aging: a systematic review. Gerontologist. 2019;59(4):E311‐E324. doi: 10.1093/GERONT/GNY108 [DOI] [PubMed] [Google Scholar]
- 81. MacBeth A, Gumley A. Exploring compassion: a meta‐analysis of the association between self‐compassion and psychopathology. Clin Psychol Rev. 2012;32(6):545‐552. doi: 10.1016/j.cpr.2012.06.003 [DOI] [PubMed] [Google Scholar]
- 82. Rebar AL, Stanton R, Geard D, Short C, Duncan MJ, Vandelanotte C. A meta‐meta‐analysis of the effect of physical activity on depression and anxiety in non‐clinical adult populations. Health Psychol Rev. 2015;9(3):366‐378. doi: 10.1080/17437199.2015.1022901 [DOI] [PubMed] [Google Scholar]
- 83. Gordon BR, McDowell CP, Lyons M, Herring MP. The effects of resistance exercise training on anxiety: a meta‐analysis and meta‐regression analysis of randomized controlled trials. Sports Medicine. 2017;47(12):2521‐2532. doi: 10.1007/s40279-017-0769-0 [DOI] [PubMed] [Google Scholar]
- 84. Conn VS. Anxiety outcomes after physical activity interventions: meta‐analysis findings. Nurs Res. 2010;59(3):224‐231. doi: 10.1097/NNR.0b013e3181dbb2f8 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


