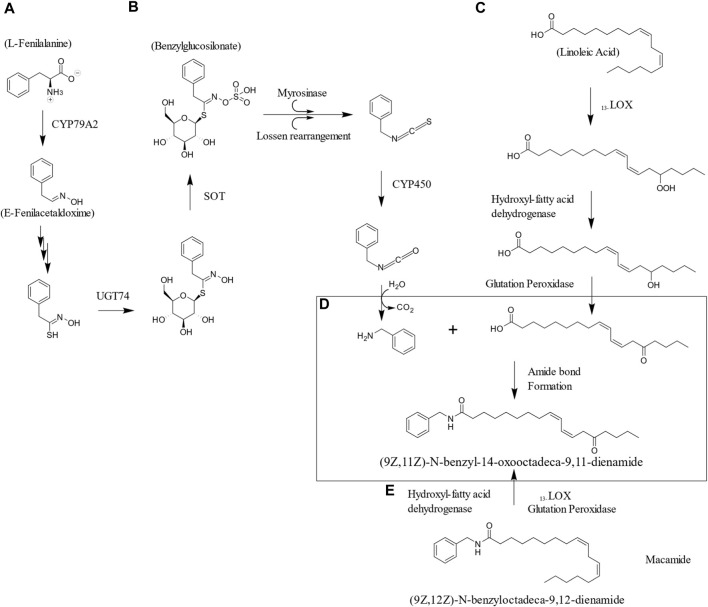
FIGURE 3.
Hypothetical biosynthetic pathway to exemplify macamides formation during natural air-drying process. (A) The biosynthesis of benzyl glucosinolate from phenylalanine comprised the formation of (E)-Phenylacetaldoxime as intermediate produced by CYP79A2. This later transform into thiohydroximate and is glycosylated and sulfated. The unstable sulfated compound leads to isothiocyanate formation by the Sulfotransferase (SOT) and Glucosyltransferasa (UGT74) (Blažević et al., 2020). (B) The enzymolysis of the benzylglucosinolate the generation of benzylamine is accomplished by (Zhang et al., 2023). (C) In this example Linoleic acid serves as the scaffold for the formation of 9-hydroperoxy-10E,12Z-octadecadienoic acid and 13-hydroperoxy-9Z,12E-octadecadienoic acid through lipoxygenase enzymes, and then transfers the fundamental skeleton of macaenes using 9 or 13-hydroperoxy-10E,12Z-octadecadienoic acid. Glutathione peroxidase and hydroxy-fatty acid dehydrogenase enzymes catalyze the conversion of 9 or 13-hydroxy-10E,12Z-octadecadienoic acid into 9 or 13-oxo-10E,12Z-octadecadienoic acid. Isomerization also produces more isomers of octadecadienoic acid, resulting in the known chemical variety of macamides (Xia, 2021). (D) Once the hydrolysis of lipids and glucosinolates release significant amounts of unsaturated free fatty acids and benzylamines. These are precursors are the core for macamide biosynthesis (Esparza, 2015). (E) Isomerization can also occur once the amide bond is formed with the unaltered linoleic acid (Xia, 2021).