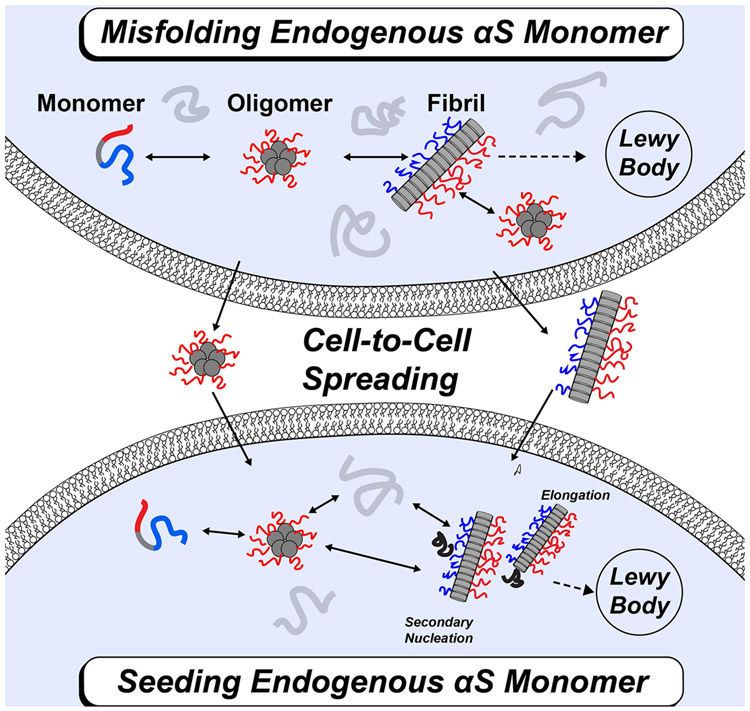
FIGURE 1: Schematic of α-Syn protein aggregation and cell-to-cell spreading.
(a) Overview of α-Syn aggregation. Endogenous α-Syn monomers (grey) can self-associate into various aggregates including dimers and oligomers. These aggregates can further go on to form different polymorphic amyloid fibrils that can be found in the Lewy bodies of patients with PD. Fibril seeds from one neuron can be released and taken up by a neighboring neuron. This cell-to-cell spreading leads to additional disease progression. (b) Mechanism of α-Syn protein fibril growth and propagation. Endogenous α-Syn monomer can interact with exogenous fibril seeds through elongation and/or secondary nucleation to form mature fibrils.