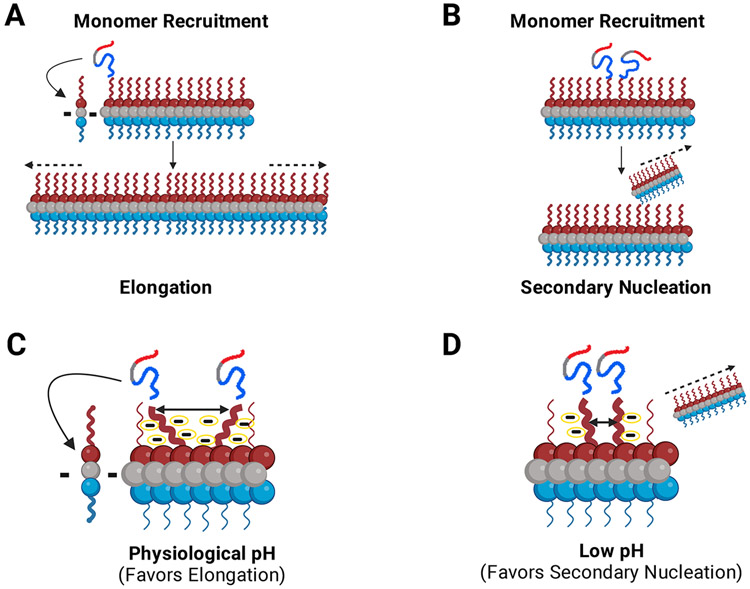
FIGURE 4: Monomers are recruited via the fuzzy coat C-terminal domain for fibril growth and propagation.
The negatively charged C-terminal domain acts as a pH dependent master regulator of the seeding process via modulation of interchain N-terminal monomer to C-terminal fibril interactions. (a, b). α-Syn monomers are recruited to the fibril via interactions between the monomer N-terminal domain and the fuzzy-coat C-terminal domain of fibrils. Incoming monomers can be integrated into the fibril through two different aggregation pathways: (a) Depiction of fibril elongation. Monomers are recruited and added onto either end of the fibril leading to elongation of the fibril. (b) Depiction of fibril secondary nucleation. Recruited monomers use the fuzzy-coat C-terminus on existing fibrils as a template for secondary nucleation. (c, d). The proposed model for the role of C-terminal charge on fibril growth. We postulate that elongation and secondary nucleation are in a constant dynamic balance. This balance can shift depending on numerous factors including pH and environmental conditions. (c) At physiological pH (~7.4), the C–terminal regions of the fibril are highly charged leading them to repel one another. Since the C-terminal regions repel each other, the incoming recruited monomers are relatively dispersed on the fibril surface and elongation is the favored mechanism of aggregation at physiological pH. (d) At low pH (~5.5-6), the charge content and distribution of the C-terminal region is changed due to the altered pKas of negatively charged residues. On average, the termini are less negatively charged and therefore, lower repulsion leads to the monomers being more proximal, productively aligned and, thus, more likely to nucleate a new fibril. The more compact nature of the complex gives rise to secondary nucleation as the dominant mechanism of aggregation at low pH. Dashes (c, d) indicate schematic representation of negative charges around the fibril C-terminal region.