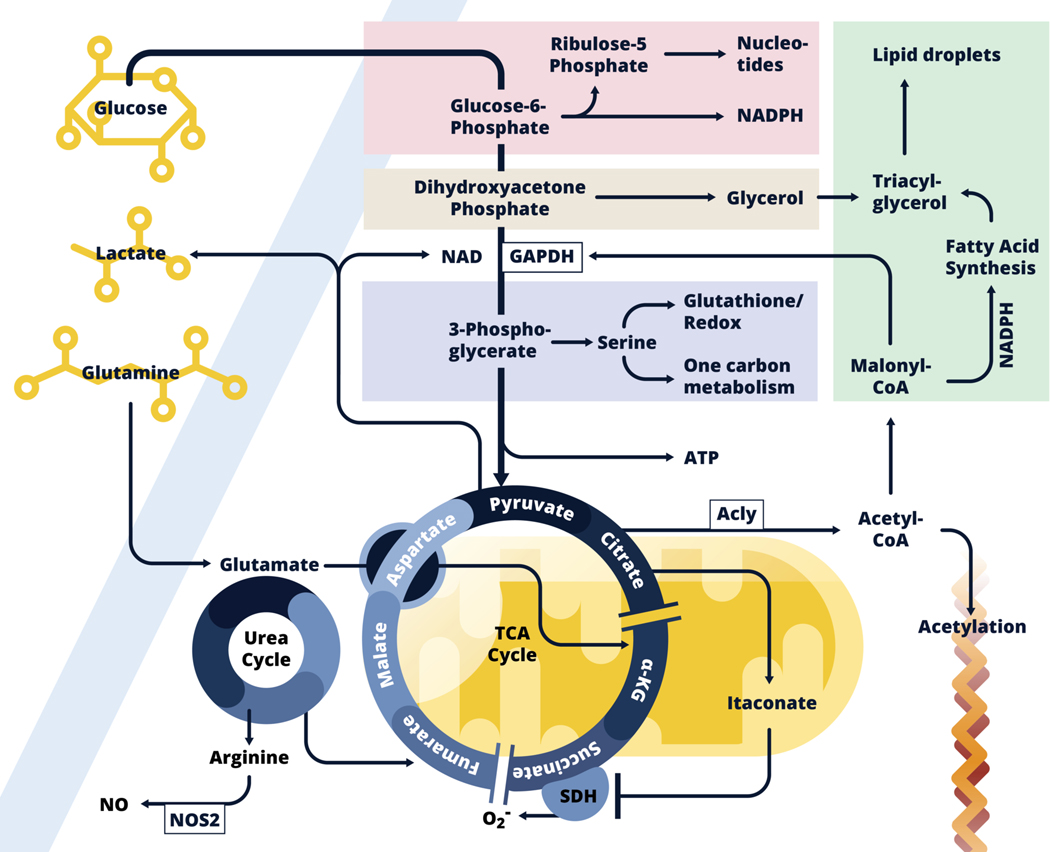
Figure 1:
Inflammatory dendritic cell and macrophage metabolism.
Glycolysis allows glucose carbon to be taken up and used not only to fuel the TCA cycle, but also to feed into the pentose phosphate pathway (Pink), the glycerol phosphate shuttle (Beige), and the serine synthesis pathway (Lavender), all of which are implicated in inflammatory activation. Core functions associated with these pathways are noted. Downstream in the glycolysis pathway, pyruvate is converted into lactate, which allows ATP generation in the absence of respiration, or enters into mitochondria (Yellow) where it is converted into acetyl-CoA which enters the TCA cycle. In inflammatory macrophages, expression of Idh, the enzyme that converts citrate to α-KG is suppressed, and citrate levels build and fuel both itaconate production within mitochondria, and following export into the cytoplasm, the production of acetyl-CoA which is used for fatty acid synthesis (FAS, Green), and as a donor for the acetylation of proteins, including histones. Fatty acids (synthesized and acquired) are used with glycerol for triacylglycerol synthesis and lipid droplet formation. Malonyl-CoA, an intermediate in the FAS pathway posttranslationally modifies GAPDH, the central enzyme in the glycolysis pathway, to minimize its RNA-binding properties thereby promoting the release and translation of Tnf mRNA. Succinate dehydrogenase (SDH), which catalyzes the conversion of succinate to fumarate, is inhibited by itaconate. Succinate accumulates and promotes inflammatory activation through reverse electron transport at Complex I to Complex II, with the accompanying generation of ROS. This leads to HIF1-α activation which promotes glycolysis. Itaconate also promotes activation of Nrf2, which allows parallel induction of anti-inflammatory effects (not shown). Induced Nos2 expression leads to the production of NO which results in the cessation of respiration due to the inhibition of components of the electron transport chain. Under these conditions, production of ATP by glycolysis is required for cell survival. Aspartate generated from glutamate, fuels the urea cycle in which nitrogens are donated to arginine, the substrate for NO production by NOS2. The urea cycle feeds the TCA cycle by donating carbons in the form of fumarate, which can generate malate, aspartate and eventually citrate. Glutamine also back-fills the TCA cycle at α-KG downstream.