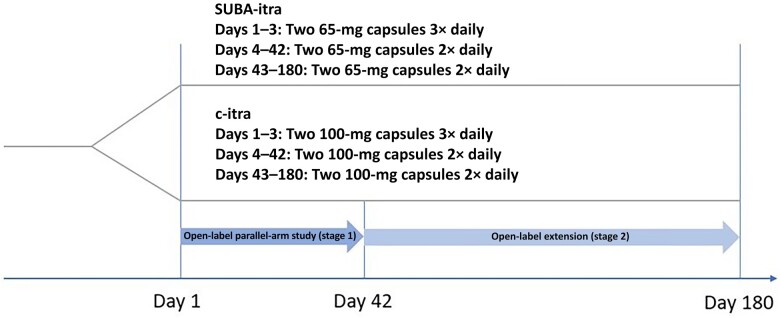
Figure 1.
Flow diagram of study design for MSG-15. Patients were enrolled into the study and randomized to super-bioavailability itraconazole (SUBA-itra) or conventional itraconazole (c-itra), followed up for 42 days in an open-label parallel-arm study (stage 1), and, if clinically indicated, further enrolled into an open-label extension (stage 2), up to 180 days.