Abstract
Feline lower urinary tract disease (FLUTD) is considered to be one of the most common diagnoses in feline patients. Several authors have concluded that feline idiopathic cystitis is the most common cause of FLUTD, whereas infectious cystitis is diagnosed in only 2% of the cases. In the period from January 2003 to February 2005, 134 cats that presented with signs of lower urinary tract disorders were included in a study at the Norwegian School of Veterinary Science. Ninety-seven percent were first opinion cases. All the cats went through a physical examination, and blood samples were collected for haematology and clinical chemistry. The urine analysis included urine stix, specific gravity, microscopic examination of the sediment and microbiological culturing. The urine samples were collected as voided mid-stream urine samples, by catheter or by cystocentesis and the method used was registered. Of the 134 cats included in the study, 37% were diagnosed as having obstructive and 63% as having non-obstructive FLUTD. In total 44 cats (33%) were diagnosed with bacteriuria, exceeding 103 colony forming units per millilitre (cfu/ml) and 33 (25%) of these cats had bacterial growth exceeding 104 cfu/ml, either alone or in combination with crystals and/or uroliths. Six cats (18%) with bacterial growth exceeding 104 cfu/ml were older than 8 years. No significant difference was found between the sampling methods performed with regard to bacteriuria. This study indicates that bacteriuria may have been underdiagnosed in Norwegian cats with clinical signs of FLUTD. It also confirms the importance of microbiological culturing in first opinion cases with FLUTD and that a skilled operator can get representative samples regardless the choice of method.
The term feline lower urinary tract disease (FLUTD) describes the collection of clinical signs caused by irritation of the mucosa of the bladder and/or urethra of cats. The presence of infectious agents, tumours, urethral plugs, uroliths, malformations and trauma can all cause similar clinical signs. In many cases no specific cause can be found and these cats are considered to have idiopathic cystitis (FIC). Cats with FLUTD typically present with signs of stranguria, dysuria, haematuria, pollakiuria and inappropriate urination; however, these signs are rarely indicative of a particular aetiology (Kruger et al 1991, Buffington et al 1997).
The majority of studies on FLUTD have been published from the USA, where the prevalence of this disease has been estimated to be 0.85% (Lawler et al 1985). In the UK a similar prevalence of 0.6% has been reported (Gaskell 1990). The true prevalence of FLUTD in first opinion practise is not known, and data from elsewhere in Europe are also lacking; however, FLUTD is considered to be one of the most common conditions affecting feline patients in the Netherlands (Kraijer et al 2003).
Several authors have concluded that FIC is the most common cause (55–69%) of FLUTD, whereas infectious cystitis has usually been diagnosed in less than 3% of cases (Kruger et al 1991, Buffington et al 1997, Kalkstein et al 1999a, b, Gunn-Moore 2003, Cameron et al 2004). However, a few studies have shown a higher prevalence of infectious cystitis (Lekcharoensuk et al 2001, Kraijer et al 2003, Gerber et al 2005), and most studies showing a low prevalence of infectious causes have been based on referral populations of cats, which may be a biased population. In an earlier student project (unpublished data) performed at the Norwegian School of Veterinary Science (NSVS), we found that 18% of the patients with FLUTD seen at our clinic were found to have significant bacteriuria – something that provoked our interest to perform the present study.
The out-patient clinic at NSVS receives about 100 cats with FLUTD each year and the majority of them are first opinion cases. The aims of this retrospective study were to report the occurrence of different types of FLUTD in cats treated at the NSVS, and to investigate whether different methods of urine collection affected the diagnosis of bacteriuria. Furthermore, we wanted to determine if there were factors that might be of potential influence on the occurrence of bacteriuria and infectious cystitis.
Materials and methods
Materials
The study population consisted of 134 client-owned cats coming from Oslo and the surrounding suburbs. They were diagnosed and treated at the Department of Companion Animal Clinical Sciences, NSVS during the period from January 2003 to February 2005.
Cats with signs of lower urinary tract disease (stranguria, dysuria, haematuria or pollakiuria) and diagnoses consistent with FLUTD (urethral plugs, urolithiasis, idiopathic cystitis, lower UTIs, bladder neoplasia, urinary incontinence, or trauma, malformations or behavioural disorders affecting the bladder) were included in the study. No breed, age, gender or other restrictions were made and cats having their first ever episode of FLUTD and those with previous episodes of FLUTD were included. Cats already receiving treatment that could interfere with the diagnostics were excluded. Cats were only included if the owner's consent was obtained.
Methods
The study design was an epidemiological descriptive study of FLUTD among cats treated at NSVS. All the cats included in the study went through a complete physical examination, and blood samples for standard haematology and clinical chemistry were collected. The urine samples were obtained either by cystocentesis, sterile catheterisation or by collecting urine voided after gentle bladder squeezing into sterile containers. Standard urinalysis was performed in all cases and included commercial urine dipstick analysis (Krulab; Kruuse, Marslev, Denmark), specific gravity measured with a refractometer (URC-Ne, ATAGO, Tokyo, Japan) and microscopic examination of the sediment (native samples and samples stained with Sternheimer–Malbins). Quantitative urine bacteriology was performed by streaking 1 μl of urine on to blood agar plates and qualitatively by cultivation from the sediment after centrifugation. The bacterial isolates from urine samples were also analysed for susceptibility to antibacterial agents on Mueller–Hinton (MH) agar (Difco, Detroit, MI) with antimicrobial discs (NeoSensitabs; Rosco, Taastrup, Denmark). The method of urine collection was noted in each case. One hundred and fourteen (85%) cats underwent an ultrasound examination of their urinary tract and further eight (6%) cats went through a radiological examination of their abdomen. Twelve (9%) cats had no imaging performed.
The climatic conditions at the time the clinical signs first started were recorded for each cat, along with information about previous/concomitant disease, feeding regime, the cat's temperament and their domestic environment (outdoor access, additional animals in the household, and type of housing), through a standardised questionnaire.
The results are expressed as prevalence in percent with 95% confidence intervals (CIs) in brackets constructed by using the theory of simple binomial sequences (Altman 1991). The assumed continuously distributed variables presented in the description of the material are given as mean values with standard deviations (SDs) in brackets. The prevalences were not considered to be significantly different from other corresponding prevalences on a significance level of 5% if a prevalence was included in the 95% CI of a corresponding prevalence or vice versa.
Results
A total of 134 cats met the inclusion criteria for this study, and of these 130 (97%) were first opinion cases and only four (3%) were referred cases. There were 86 (64%) castrated males, 21 (16%) intact males, 19 (14%) spayed females and eight (6%) intact females. One hundred and fourteen (85%) cats were Norwegian domestic short-, semi- or long-haired cats, the rest was Norwegian Forest cats (n=8; 6%), Persian cats (n=7; 5%), Maine Coons (n=3; 2%) and Abyssinians (n=2; 1%). The mean age of the cats was 5.7 years (SD 3.7) and mean weight was 5.0 kg (SD 1.3).
Background of the cats
Ninety-two (69%) cats had experienced previous episodes of FLUTD. Sixty-eight (51%) cats were indoor-only cats, 56 (42%) had outdoor access while the status of the remaining 10 (7%) cats was not registered. The onset of clinical signs was divided up by the season–46 (34%) cases occurred in the winter, 36 (27%) in the spring, 23 (17%) in the summer and 29 (22%) in the autumn. The type of weather was also registered with 77 (58%) cases occurring during periods of dry weather, and 41 (31%) in periods of more humid/wet weather. For the remaining 15 (11%) the weather conditions were not registered (Table 1).
Table 1.
Summary of the occurrence of FLUTD and bacteriuria>104 cfu/ml with regard to the various factors of potential influence
| Potentially influencing factors | FLUTD cases (%) | Bacteriuria (%) (95% CI) |
|---|---|---|
| Season | ||
| Winter | 45 (34) | 12 (27) (16–41) |
| Spring | 36 (27) | 9 (25) (14–41) |
| Summer | 23 (17) | 8 (35) (19–55) |
| Autumn | 30 (22) | 4 (13) (5–30) |
| Climate/weather | ||
| Dry weather | 77 (57) | 15 (20) (12–30) |
| Humid weather | 42 (31) | 13 (31) (19–46) |
| Not registered | 15 (11) | |
| Environment | ||
| Indoor | 69 (51) | 19 (28) (19–39) |
| Outdoor/both | 56 (42) | 12 (21) (13–34) |
| Not registered | 9 (7) | |
| Weight | ||
| <3 kg | 8 (6) | 4 (50) (22–78) |
| 3–5 kg | 61 (46) | 13 (21) (13–33) |
| 5–7 kg | 53 (40) | 15 (28) (18–42) |
| >7 kg | 7 (5) | 0 |
| Not registered | 5 (4) | |
| Gender | ||
| Male | 21 (16) | 7 (33) (17–55) |
| Castrated male | 86 (64) | 18 (21) (14–31) |
| Female | 8 (6) | 4 (50) (22–78) |
| Neutered female | 19 (14) | 4 (21) (9–43) |
| Age | ||
| <1 Year | 6 (4) | 3 (50) (19–81) |
| 1–4 Years | 54 (40) | 11 (20) (12–33) |
| 4–8 Years | 46 (34) | 11 (24) (14–38) |
| 8–12 Years | 19 (14) | 5 (26) (12–49) |
| >12 Years | 5 (4) | 1 (20) (4–62) |
FLUTD=feline lower urinary tract disease.
Urinary findings
Of the 134 cats included in the study, 37% (CI: 32–42%) were diagnosed with obstructive FLUTD and 63% (CI: 57–69%) with non-obstructive disease. The findings from the examination of the urinary sediment and the ultrasound or radiographic examination of the urinary bladder are listed in Table 2. This paper will focus on the cats with bacteriuria with or without other findings in the urine or urinary bladder.
Table 2.
Distribution of bacteria (>104 cfu/ml), crystals and uroliths in the urine of 134 cases with FLUTD
| Urinary findings | Obstructive FLUTD | Non-obstructive FLUTD |
| Uroliths | 3 (6%) | 1 (1%) |
| Crystals | 10 (20.5%) | 31 (36.5%) |
| Uroliths and crystals | 13 (26.5%) | 2 (2.5%) |
| Bacteriuria | 2 (4%) | 14 (16.5%) |
| Uroliths and bacteriuria | 2 (4%) | 1 (1%) |
| Crystals and bacteriuria | 2 (4%) | 8 (9.5%) |
| Uroliths, crystals and bacteriuria | 3 (6%) | 1 (1%) |
| Bacteria, crystals or uroliths not found | 14 (29%) | 27 (32%) |
| Total | 49 (100%) | 85 (100%) |
The urinary findings are based on microscopic examination of the urinary sediment, microbiological culturing and imaging (114 cats had ultrasound of the bladder performed, eight cats had radiography performed and 12 cats had no imaging performed).
Bacteriuria
The method of urine sampling for each cat was voided mid-stream urine in 62 cases (46%), via sterile urinary catheter in 28 (21%) by cystocentesis in 30 (22%) and in 14 cases (10%), the method was not registered. Cats that went through catheterisation or cystocentesis were sedated with a combination of medetomidin (Domitor vet; Orion, Turku, Finland) 30 μg/kg SC and methadone hydrochloride (Metadon inj; Rikshospitalets apotek, Oslo, Norway) 0.2 mg/kg SC.
In 118 cases, the urine sample was cultured on the same day that it was collected. From these samples, 44 yielded a bacterial growth exceeding 103 colony forming units per millilitre (cfu/ml). In 33 of these 44 samples the bacterial growth exceeded 104 cfu/ml, and in 20 samples the bacterial growth exceeded 105 cfu/ml with seven samples showing close to 106 cfu/ml urine. None of the 16 samples cultured the day after collection (or with unknown date of sampling) produced bacterial growth exceeding 103 cfu/ml. There was no bacterial growth from the urine samples of 69 (51%) cats (Table 3).
Table 3.
Results from bacteriological cultivation
| Bacterial species | Rich (>104 cfu/ml) | Moderate (103–104 cfu/ml) | Sparse (<103 cfu/ml) | Overall bacterial growth |
| Escherichia coli | 14 | 6 | 5 | 25 (38.5%) |
| Streptococcus canis | 2 | 1 | 3 (4.6%) | |
| Staphylococcus species | 2 | 2 | 4 (6.2%) | |
| Enterococcus species | 1 | 1 | 2 (3.0%) | |
| Streptococcus, ß-haemolytic | 1 | 1 (1.5%) | ||
| Staphyloccoccus intermedius | 1 | 1 (1.5%) | ||
| Hemophilus species | 1 | 2 | 3 (4.6%) | |
| Proteus species | 1 | 1 (1.5%) | ||
| Enterobacter species | 1 | 1 (1.5%) | ||
| Enterobacter cloacae | 1 | 1 (1.5%) | ||
| Pasteurella species | 3 | 3 (4.6%) | ||
| Citrobacter species | 1 | 1 (1.5%) | ||
| Staphylococcus aureus | 1 | 1 (1.5%) | ||
| Combination of the bacteria above | 8 | 1 | 9 | 18 (27.6%) |
| Total | 33 | 11 | 21 | 65 (100%) |
Cfu/ml=colony forming units per millitre
Of the 33 cases with bacterial growth exceeding 104 cfu/ml the most common findings were Escherichia coli from 14 (42.5%) of the cases, Streptococcus canis (n=2; 6%), and Staphylococcus species (n=2; 6%). The rest of the bacteriological results is listed in Table 3.
The percentages of cats without and with bacteriuria exceeding 104 cfu/ml were compared for the three methods of urine collection. Bacteriuria was identified in 26% (CI: 17–38%) of the samples collected as voided mid-stream urine, in 23% (CI: 12–41%) of the samples collected by cystocentesis and in 14% (CI: 6–31%) of the samples collected by catheterisation. There was no significant difference in the prevalence of bacteriuria between these three techniques (Fig 1).
Fig 1.
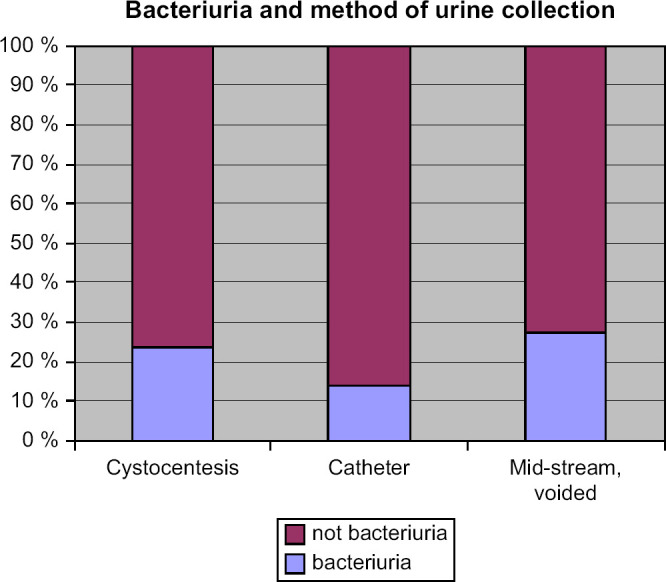
The percentages of cats without and with bacteriuria>104 cfu/ml that were registered for the three methods of urine collection.
Twelve percent (CI: 7–29%) of the cats with obstructive FLUTD had bacteriuria exceeding 104 cfu/ml, alone or in combination with crystals and/or uroliths. Only 2% (-2-6%) of these cats had bacteriuria alone. The corresponding percentages for the cats with non-obstructive FLUTD were 28% (18–38%) with bacteriuria alone or in combination with crystals and/or uroliths and 17% (8–24%) with bacteriuria alone. In total 25% (20–30%) of the cats were diagnosed with bacteriuria, and 11% (7–15%) with bacteriuria alone.
There were no significant differences between the variables: season, climatic conditions, domestic environment, gender, age and weight, although some strong tendencies could be observed. Furthermore, it should be stressed that the size of some of the groups makes statistical interpretation difficult.
Table 1 shows the breakdown of cats with FLUTD and with bacteriuria exceeding 104 cfu/ml according to season, climate, environment, weight, age, and gender. Although the summer months had the lowest total number of FLUTD cases recorded, this period had the highest relative proportion of bacteriuria cases (35%) followed by the winter, the spring and the autumn. Also a greater relative proportion of bacteriuria cases were seen in humid (31%) compared with dry (20%) conditions. Looking at the gender of the cats, entire females had the highest relative proportion of bacteriuria (50%), followed by intact males (33%). Bacteriuria was also relatively more common in young cats (cats of <1 year of age had a relative prevalence of bacteriuria of 50%) compared to older cats, and in cats weighing <3 kg (50%) compared with heavier cats, although there were relatively few cats that were <1 year or >12 years of age, or those weighing <3 kg or >7 kg.
Discussion
This paper reports a study of 134 cases with FLUTD in Norway. Bacteriuria exceeding 103 cfu/ml was detected in 44 cats (33%) and 33 of these cats had bacterial growth exceeding 104 cfu/ml, a finding that deviates from the results in most previously published studies where less than 2% of the FLUTD cases were found with bacteriuria (Kruger et al 1991, Buffington et al 1997, Kalkstein et al 1999a, b, Gunn-Moore 2003, Cameron et al 2004).
In the retrospective study of risk factors for lower urinary tract diseases in cats performed by Lekcharoensuk et al (2001), 12% of the cats were diagnosed with a UTI. This was a case control study and included 22,908 cats with lower urinary tract disease and 263,168 cats without. The data were retrieved from medical records for all cats evaluated at veterinary teaching hospitals in the US and Canada that submitted data to the Veterinary Medical Data Base between 1980 and 1997. However, because of the retrospective nature of that study, it was not possible to determine the extent of diagnostic evaluation for each patient which makes the results less reliable. In another study, performed in the Netherlands by Kraijer et al (2003), 25% of 32 primary FLUTD cases selected by veterinary practitioners had a UTI, a finding which is similar to the current study. The authors concluded that their results may have differed from other studies on the aetiology of FLUTD because other studies were performed at university hospitals, thus focusing on a pre-selected population of referred cases. This problem is also discussed in a paper published by Gerber et al (2005). Veterinary colleges and schools in the USA and Europe mostly receive referred cases and they miss the opportunity to examine and investigate untreated primary cases with FLUTD. Referred cases may previously have received treatment that could potentially camouflage the initial problem. The results of the studies performed on referred cases may, therefore, have been biased towards a different population of cases. The fact that the NSVS receives mainly first opinion cases may contribute to the large proportion of cats with bacteriuria as primary and less complicated cases normally treated at local veterinary clinics also were included.
Several authors have claimed that most cats with bacteriuria are older cats because older cats have an increased risk of concurrent diseases; such as diabetes mellitus or chronic renal failure that makes them more susceptible to infections (Lees 1996, Hostutler et al 2005). In our study there were 24 cats older than 8 years and of those, six had bacteriuria and none of them with any concurrent disease.
The gender distribution of cats is equal in our clinical population but still there were 80% males in our study. One explanation for this overrepresentation may be that there were 49 obstructed cats and only two of them were females. Because obstructed cats are an emergency, the owners consult the veterinarian as soon as they are aware of the situation. In contrast, female cats with idiopathic cystitis may hide their signs if they are outdoor cats and recover without the owner's knowledge and are, therefore, never seen by the veterinarian (Kruger et al 1996, Lekcharoensuk et al 2001).
There are probably differences in the lifestyles of cats in Norway as well as many other countries in Europe and the USA. Even in the cities, many cats in Europe lead a relatively free existence, being free to roam outdoors and to hunt mice and birds. In Norway, the cats are generally fed with seafood and meat products as a supplement to commercial diets and, as many cats dislike the taste of tap water with chlorine in it, an outdoor life allows the cats to drink ‘free water’ from ponds and flower bowls kept outside. It is possible that most of the cats represented in the studies from USA are indoor cats living in cities. These cats are probably fed primarily dry commercial diets and may not drink as much as they should due to unpalatable tap water (Cannon and Forster-van Hiijfte 2006). In addition, indoor cats may get insufficient exercise, so obesity is more likely to be a problem (Cannon and Forster-van Hiijfte 2006). Taking into account the potentially stressful situation of being an indoor cat, being obese, and having access to only dry diets and suboptimal water intake, it is possible that there could be a higher occurrence of idiopathic cystitis and urethral plugs among cats living in the cities of the USA than in Norway and maybe also the rest of Europe (Gunn-Moore 2003, Cameron et al 2004, Hostutler et al 2005).
Even though 51% of the cats in this study were indoor-only cats, the majority of cats in Norway have outdoor access (Norske Rasekattklubbers Riksforbund 2007). The overrepresentation of indoor cats in this study may indicate that FLUTD occurs more often and/or is more often detected and diagnosed in indoor cats than in outdoor cats. Furthermore, 22% of the cats in this study were intact males and females which tend to roam over larger territories than neutered cats and this may influence the owner's control of them and delay the detection of the first clinical signs of urinary disease. In addition, it is possible that they may develop infections secondary to mating behaviour. The total number of feline patients treated at the clinic was evenly distributed throughout the study period but the occurrence of FLUTD was greater in the winter time compared to the summer (although this difference was not significant). However, a larger relative proportion of cats were diagnosed with bacteriuria in the summer compared to other seasons (although again this was not a significant difference). In the summer, the cats may make much more use of their outdoor access compared to the winter, when they may lead an existence more similar to the indoor cats. This may perhaps constitute the outlines of a pattern; a more active outdoor life in the summer, with a higher risk of infectious cystitis due to the often cold and humid weather conditions and more time out of the owner's observation, and a less active indoor life in the winter with FLUTD cases more dominated by idiopathic cystitis and obstructive plugs (Jones et al 1997, Cameron et al 2004). However, further epidemiological studies, incorporating good control populations would be needed to explore these findings further.
Cystocentesis is considered to be the method of choice when urine is sampled for microbiological evaluation (van Duijkeren et al 2004). However, in some situations cystocentesis is impractical. For example, in cases without obstruction, some cats continuously void small amounts of urine due to inflammation in the urinary bladder, and, therefore, keep their bladder empty. It can, thus, be difficult and time consuming to obtain a representative urine sample for microbiological analyses, so bacterial culture may be omitted by some veterinarians. As many samples are obtained by catheter or as voided samples, it was interesting to determine whether these samples could be used reliably for the diagnosis of clinically significant bacteriuria. It is important to note that the urine samples in this study were almost all cultured on the day of collection. There were 69 (51%) urinary samples with no bacterial growth and these samples were equally distributed between the three sampling methods indicating that sampling was optimally performed with minor contamination from urethra, skin and hair. As a result, samples with cfu/ml urine exceeding 103 could be included in the group of samples defined to be from cats with bacteriuria making the number of cats with bacteriura to be 44 (33%) (Table 3). Lekcharoensuk et al (2001), Kraijer et al (2003) and Gerber et al (2005) did not report a cut-off value for bacterial infection in cats but this was given by Wooley and Blue (1976), who used 105 cfu/ml as a cut-off value. In cultivation of urine from humans cfu/ml>105 has been considered a reasonable level at which to determine that a person has significant bacteriuria (Bonadio et al 2006). This cut-off level will include both samples from individuals with asymptomatic bacteriuria and with true UTI. Except for the study of Wooley and Blue (1976), there is minimal evidence supporting the use of the same cut-off level for cfu/ml urine from cats with UTI as when culturing of samples from humans. Our study indicates that for urine samples of cats with FLUTD, a cfu/ml of >103 may be indicative of bacteriuria causing or potentially causing UTI. In 2004, van Duijkeren et al found that numbers of bacteria were low (102–103 cfu/ml) in three out of eight culture-positive samples taken by cystocentesis from cats with acute FLUTD. The authors conclude that this indicates that the number of bacteria present in the bladder of cats with UTIs may be low. Subsequently they state that this situation may lead to underdiagnosis of UTIs when interpreting culture results for voided and catheterised samples, because bacterial counts below 103 cfu/ml urine are generally considered not clinically relevant.
No significant difference was found between the sampling methods with regard to bacteriuria in this study. The fact that the samples in this study were analysed at the veterinary school with no delay due to transportation might be of importance, as the growth of contaminating bacteria is prevented. This study does indicate that if great care is taken with regards to hygiene, a skilled operator can get representative samples regardless the choice of method.
The species distribution among the bacterial isolates from the feline urine samples is in accordance with what has been previously published (Wooley and Blue 1976), with E coli being the dominant organism, as in dogs and humans. Some studies have recently demonstrated that the E coli isolates of urine from cats and dogs belong to the same serotypes and possess the same virulence factors as urinary tract isolates from humans (Yuri et al 1998, 1999, Feria et al 2001, Kurazono et al 2003). It has been speculated that canine and feline E coli strains may represent a zoonotic potential (Kurazono et al 2003).
The results of this study indicate that bacteriuria may have been underdiagnosed and underrecognised in Norwegian cats, as most veterinarians have based their knowledge about FLUTD on the results from previously published papers on this subject from other countries. The importance of microbiological culturing in first opinion cases is confirmed by this present study and further research is needed to establish the prevalence of the causes of FLUTD in Europe and the USA.
Acknowledgements
The authors would like to thank all our colleagues at the Norwegian School of Veterinary Science who have contributed to this study.
References
- Altman D.G. Practical Statistics for Medical Research, 1991, Chapman & Hall: London. [Google Scholar]
- Bonadio M., Costarelli S., Morelli G., Tartaglia T. The influence of diabetes mellitus on the spectrum of uropathogens and the antimicrobial resistance in elderly adult patients with urinary tract infection, BMC Infectious Diseases 6, 2006, 54–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffington C.A.T., Dennis J.C., Kendall M.S., Scrivani P.V., Thompson S.B., Blaisdell B.S., Woodworth B.E. Clinical evaluation of cats with non-obstructive urinary tract diseases, Journal of the American Veterinary Medical Association 210, 1997, 46–50. [PubMed] [Google Scholar]
- Cameron M.E., Casey R.A., Bradshaw J.W.S., Waran N.K., Gunn-Moore D.A. A study of environmental and behavioural factors that may be associated with feline idiopathic cystitis, Journal of Small Animal Practice 45, 2004, 144–147. [DOI] [PubMed] [Google Scholar]
- Cannon M., Forster-van Hiijfte M. Feline Medicine, A Practical Guide for Veterinary Nurses and Technicians, 2006, Elsevier Butterworth Heinemann: Edinburgh, 39–54. [Google Scholar]
- van Duijkeren E., van Laar P., Houwers D.J. Cystocentesis is essential for reliable diagnosis of urinary tract infections in cats, Tijdschrift voor Diergeneeskunde 129, 2004, 394–396. [PubMed] [Google Scholar]
- Feria C., Machado J., Correia J.D., Goncalves J., Gaastra W. Virulence genes and P fimbriae PapA subunit diversity in canine and feline uropathogenic Escherichia coli, Veterinary Microbiology 82, 2001, 81–89. [DOI] [PubMed] [Google Scholar]
- Gaskell C.J. Feline urological syndrome (FUS) – theory and practice, Journal of Small Animal Practice 31, 1990, 519–522. [Google Scholar]
- Gerber B., Boretti F.S., Kley S., Laluha P., Müller C., Sieber N., Unterer S., Wenger M., Flückiger M., Glaus T., Reusch C.E. Evaluation of clinical signs and causes of lower urinary tract disease in European cats, Journal of Small Animal Practice 46, 2005, 571–577. [DOI] [PubMed] [Google Scholar]
- Gunn-Moore D.A. Feline lower urinary tract disease, Journal of Feline Medicine and Surgery 5, 2003, 133–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hostutler R.A., Chew D.J., DiBartola S.P. Recent concepts in feline lower urinary tract disease, Veterinary Clinics of North America. Small Animal Practice 1, 2005, 147–170. [DOI] [PubMed] [Google Scholar]
- Jones B.R., Sanson R.L., Morris R.S. Elucidating the risk factors of feline lower urinary tract disease, New Zealand Veterinary Journal 45, 1997, 100–108. [DOI] [PubMed] [Google Scholar]
- Kalkstein T.S., Kruger J.M., Osborne C.A. Feline idiopathic lower urinary tract disease. Part I. Clinical manifestations, Compendium on Continuing Education for the Practicing Veterinarian 21, 1999a, 15–26. [Google Scholar]
- Kalkstein T.S., Kruger J.M., Osborne C.A. Feline idiopathic lower urinary tract disease. Part II. Potential causes, Compendium on Continuing Education for the Practicing Veterinarian 21, 1999b, 148–154. [Google Scholar]
- Kraijer M., Fink-Gremmels J., Nickel R.F. The short-term clinical efficacy of amitriptyline in the management of idiopathic feline lower urinary tract disease: a controlled clinical study, Journal of Feline Medicine and Surgery 2005, 2003, 191–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruger J.M., Osborne C.A., Goyal S.M., Wickstrom S.L., Johnston G.R., Fletcher T.F., Brown P.A. Clinical evaluation of cats with lower urinary tract disease, Journal of the American Veterinary Medical Association 199, 1991, 211–216. [PubMed] [Google Scholar]
- Kruger J.M., Osborne C.A., Lulich J.P. Management of nonobstructive idiopathic feline lower urinary tract disease, Veterinary Clinics of North America. Small Animal Practice 26, 1996, 571–588. [DOI] [PubMed] [Google Scholar]
- Kurazono H., Nakano M., Yamamoto S., Ogawa O., Yuri K., Nakata K., Kimura M., Makino S., Nair G.B. Distribution of the usp gene in uropathogenic Escherichia coli isolated from companion animals and correlation with serotypes and size-variations of the pathogenicity island, Microbiology and Immunology 47, 2003, 797–802. [DOI] [PubMed] [Google Scholar]
- Lawler D.F., Sjolin D.W., Collins J.E. Incidence rates of feline lower urinary tract disease in the United States, Feline Practice 15, 1985, 13–16. [Google Scholar]
- Lees G.E. Bacterial urinary tract infections, Veterinary Clinics of North America. Small Animal Practice 26, 1996, 297–304. [PubMed] [Google Scholar]
- Lekcharoensuk C., Osborne C.A., Lulich J.P. Epidemiologic study of risk factors for lower urinary tract diseases in cats, Journal of the American Veterinary Medical Association 218, 2001, 1429–1435. [DOI] [PubMed] [Google Scholar]
- Norske Rasekattklubbers Riksforbund (2007) 3482 Tofte, Norway (also member of Fédération Internasjonale Féline (FIFe)). Personal communication.
- Wooley R.E., Blue J.L. Quantitative and bacteriological studies of urine specimens from canine and feline urinary tract infections, Journal of Clinical Microbiology 4, 1976, 326–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuri K., Nakata K., Katae H., Tsukamoto T., Hasegawa A. Serotypes and virulence factors of Escherichia coli strains isolated from dogs and cats, Journal of Veterinary Medical Science 61, 1999, 37–40. [DOI] [PubMed] [Google Scholar]
- Yuri K., Nakata K., Katae H., Yamamoto S., Hasegawa A. Distribution of uropathogenic virulence factors among Escherichia coli strains isolated from dogs and cats, Journal of Veterinary Medical Science 60, 1998, 287–290. [DOI] [PubMed] [Google Scholar]


