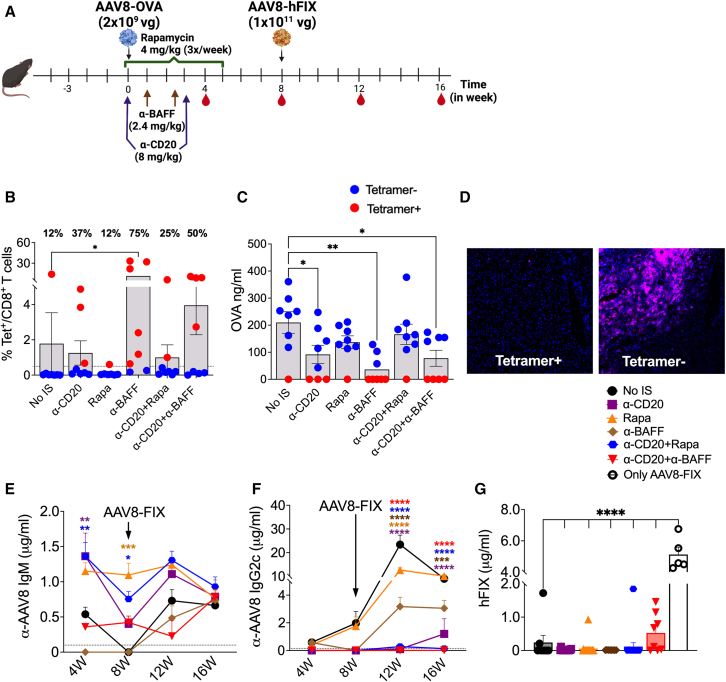
Figure 1.
Effect of concurrent IS treatment on transgene and AAV8-specific immune responses
(A) Schematic outline of IS treatments and AAV8 dosing. (B) CD8+ T cell responses to the OVA transgene quantified using MHC-I H2-Kb-SIINFEKL tetramer for various IS treatments (n = 5–8/group). Red dots indicate (Tet+) animals and blue dots indicate (Tet−) animals. Frequency of Tet+ animals in each group is indicated above each bar. (C) Circulating plasma levels of OVA at 4 weeks post-AAV8-OVA administration. (D) Representative images of OVA staining in liver tissue from mice with or without CD8+ T cell response to OVA (20× magnification). (E) Longitudinal analysis of AAV8 capsid-specific IgM Ab levels in plasma samples post primary and -secondary AAV8 administrations. The dotted line represents baseline IgM levels averaged from 8 pre-bled animals. (F) AAV8 capsid-specific IgG2c Ab levels in plasma samples. The dotted line represents baseline IgG2c levels averaged from 8 pre-bled animals. (G) Circulating human FIX levels at 4 weeks post-AAV8-hFIX administration. Statistical significance was calculated by one-way ANOVA (Dunnett’s multiple comparisons) for (B), (C), and (G), and two-way ANOVA for (E) and (F). ∗p ≤ 0.05; ∗∗p ≤ 0.01; ∗∗∗p ≤ 0.001; ∗∗∗∗p ≤ 0.0001.